Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2021
Table of contents
- Executive summary
- Use of nuclear substances in Canada: 2021
- Appendix A: Regulatory program for the use of nuclear substances
- Appendix B: Compliance performance
- Appendix C: Enforcement actions issued in 2021
- Appendix D: Doses to workers
- Appendix E: Reportable events
- Appendix F: Inspections conducted in 2021
- Appendix G: Stakeholder engagement activities in 2021
- Appendix H: Blank inspection worksheet
- Appendix I: Safety performance rating levels
- Appendix J: Relevant documents
Executive summary
The Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2021 provides information on the use of nuclear substances in the medical, industrial, academic and research, and commercial sectors. Licensees covered by this report are, for the most part, regulated by the CNSC’s Directorate of Nuclear Substance Regulation. The regulatory oversight report (ROR) also includes select waste nuclear substance licensees that are not reported on in other RORs and that are regulated by the Directorate of Nuclear Cycle and Facilities Regulation. Licensees covered by this report are located across Canada, and CNSC staff acknowledge all treaties and traditional territories on which the licensees that are subject to this report are situated.
The key message conveyed in this year’s ROR continues to be the impact of the COVID-19 pandemic on regulatory oversight, requiring the CNSC to balance the need for in-person compliance activities with the health and safety of the public and of CNSC and licensee staff. While 2021 allowed for the return of in-person inspections at a higher frequency than in 2020, the number of inspections conducted was still lower than in the years prior to the pandemic. Despite the reduced number of inspections, the indicators presented in this report demonstrate that the risks posed by the use of nuclear substances in Canada continue to be managed appropriately.
While overall compliance remains high, staff are beginning to see a moderate downward trend in some sectors for certain safety and control areas (SCAs). In particular, there was a decline in ratings this past year for the radiation protection SCA in the academic and research sector and for the security SCA in the medical sector. As explained in the 2020 ROR, CNSC staff had anticipated this decline given the restrictions on in-person inspections over the past 2 years. The link between inspections and this decline is twofold. First, inspections are a powerful tool for compliance promotion. Therefore, a lower frequency of inspections increases the probability of non-compliances by the licensee. Second, the nature of the CNSC’s risk-informed compliance program leads staff to focus their inspection effort on those licensees with the greatest potential for compliance problems, thereby introducing a bias in the performance results.
Based on their assessment of licensee performance results for 2021, CNSC staff continue to conclude that nuclear substances in Canada are used safely. However, staff will continue to monitor performance and will make it a priority to steadily increase their onsite presence. At the same time, the CNSC has a number of other ways to ensure safety across the regulated sectors, including a strong approach to licensing, the ability to react quickly to unforeseen events, and graduated enforcement tools that enable staff to bring a licensee back into compliance in cases of unacceptable performance. In addition, the CNSC’s risk-informed inspection planning process allows staff to target those licensees that are the highest priority, thereby ensuring that the CNSC’s inspector resources are applied with maximum effectiveness.
The CNSC’s annual RORs continue to evolve in response to feedback received from the Commission and from intervenors. As a result, staff have included 2 new sections in this year’s ROR: an explanation of the risk-informed planning process mentioned above, and an overview of how the CNSC assesses the environmental impact of nuclear substance licensees. In addition, a section has been reintroduced describing how the CNSC fulfills its international commitments for the 4 sectors covered in this report. This demonstration of responsiveness and transparency is a key element of the CNSC’s commitment to building trust in the nuclear regulator.
Based on the CNSC’s regulatory oversight, flexibility, and actions to respond to the changing work environment, the evaluations presented in this report confirm the following:
- The use of nuclear substances and prescribed equipment in Canada remains safe and secure.
- A rigorous process is in place to plan inspections on a priority basis.
- Thorough assessments of licensee applications ensure that licensees are compliant with regulatory requirements and understand their responsibilities.
- Doses to workers remain low and below prescribed limits.
- Non-compliances are addressed, and enforcement actions are closed in a timely manner.
- Events are reported and reviewed appropriately.
Overall, licensees made acceptable provisions to protect health, safety, security and the environment with respect to the use of nuclear substances and prescribed equipment, and took the measures required to implement Canada’s international obligations and commitments.
Use of nuclear substances in Canada: 2021
The Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2021 summarizes the safety performance of 1,500 licensees, which hold a total of 2,097 licences. The Canadian Nuclear Safety Commission (CNSC) authorizes licensees to use nuclear substances and prescribed equipment in the medical, industrial, academic and research, and commercial sectors. For a description of the licensed activities covered in this report, refer to the technical briefing to the Commission on nuclear substances in Canada (CMD 18-M49). This regulatory oversight report (ROR) also includes 5 waste nuclear substance licensees (WNSLs) that are not captured in any other CNSC ROR. They are included in the commercial sector throughout the report. Additional data on licensees covered by this ROR is available in appendix A.
CNSC staff use many metrics to evaluate licensees’ safety and security performance. This ROR uses a subset of those metrics, which, when taken together, provide a well-rounded picture of the performance of licensees. The metrics used in this report are:
Licensing oversight also plays a key role in supporting licensee performance. Rigorous assessments of licensee programs, along with timely desktop reviews of annual reports and changes submitted by the licensee, are critical in ensuring that licensees have appropriate programs and people in place to ensure the safe and secure use of nuclear substances in Canada.
In addition to the standard review of performance indicators, the 2021 ROR includes an overview of how inspection planning is used to mitigate the risk that has been introduced as a result of the decrease in inspections performed. This overview is presented in section 2.0.
In response to intervenor feedback, additional information has been included on inspection criteria (appendix H) and on environmental protection, as it relates to most licensees covered by this ROR (section 3.7). The section on international commitments and obligations has also been reintroduced in the report in response to feedback (section 8.0).
Lastly, the report provides the Commission with information about stakeholder engagement, which is a critical element of the CNSC’s regulatory approach. Given the breadth of licensees regulated in the area of nuclear substances, particular focus has been placed on reaching out to and engaging with licensee communities.
This ROR includes data in both the body and appendices. The main body of the report provides a high-level overview of the CNSC’s regulatory efforts and the licensees’ performance, while the detailed data to support this overview is found in the appendices.
1.0 Inspection overview in 2021
The COVID-19 pandemic continued to have an impact on regulatory oversight in 2021, requiring the CNSC to balance regulatory oversight with the health and safety of CNSC and licensee staff. Inspections in 2021 included a mix of remote, in-person and hybrid inspections depending on the restrictions in place at the time and the comfort level of staff in making an in-person visit to a licensee site. Until mid-2021, occupational health and safety protocols put in place in response to provincial and federal guidelines limited travel and in-person activities; however, when warranted by licensee performance, staff did perform in-person inspections. As restrictions started to ease and more routine, in-person inspections resumed in the summer, differing risk tolerances for travel and for in-person activities (for both the CNSC as a whole and for individual staff members) were key considerations when determining whether an inspection would be done in person or remotely. In fall 2021 (specific timelines dependent on location in Canada), the prevalence of new cases of the Omicron variant of COVID-19 across the country put a halt to routine onsite inspections once again.
In 2021, staff performed 583 inspections (233 in-person, 9 hybrid, and 341 remote). Most of these inspections were done in the industrial and medical sectors (63% and 27% respectively). This is to be expected as these 2 sectors make up approximately 79% of all licences and account for many of the licensed activities categorized as higher risk.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
The 583 inspections performed in 2021 exceeded the planned number of 495 inspections. It was also an increase over 2020, when only 371 inspections were performed (although it was still short of the number of annual baseline inspections conducted pre-pandemic). With the pandemic once again limiting onsite inspections, the use of remote inspections continued to be an essential component of regulatory oversight in 2021, with 60% of inspections being done remotely or in a hybrid manner. While remote inspections are a useful tool, CNSC staff believe that, in most cases, onsite inspections are the preferred option when possible; therefore, the proportion of onsite inspections is expected to increase as COVID restrictions continue to ease.
While the number of annual inspections has increased since 2020, CNSC staff can see a correlation between the reduced number of inspections due to the pandemic and the compliance results from the past 2 years (see section 3.0 for a discussion of compliance performance in 2021). The inspection planning process used by CNSC staff is designed to consider such changes in performance, using a risk-informed approach that prioritizes the most critical inspections. This planning process allows CNSC staff to maintain assurance that the nuclear substances sector continues to be safe despite the lower number of inspections (section 2.0 provides an overview of the CNSC’s inspection planning and prioritization process).
Notwithstanding these methods of mitigating the risk introduced by fewer inspections, CNSC staff will be working to increase the number of annual inspections in order to regain the baseline inspection frequency determined by the CNSC’s risk-informed compliance program. Primary responsibility for safety lies with the licensee; therefore, the CNSC expects licensees to comply with regulatory requirements whether they are subject to a CNSC inspection or not. Nonetheless, inspections are an important component of regulatory oversight that allow the CNSC to verify licensee compliance with those requirements. Moreover, they provide an opportunity for inspectors to intervene early with licensees when performance starts to decline, whether from complacency, lack of understanding of regulatory requirements, or poor safety culture. Inspections also provide a unique opportunity to gather certain types of performance information – inspectors can observe workers while they perform their duties to see if they are following procedures, and can get a sense of the overall effectiveness of a licensee’s safety culture and radiation protection program. This is especially true for licensees engaged in field work where it is important that workers follow procedures to ensure their safety, since physical barriers are not always available. CNSC staff can then use this information to make risk-informed licensing decisions.
Given the importance of inspections generally, and the priority given to addressing the current backlog, the availability of a sufficient number of trained inspectors is a key program constraint that CNSC staff are monitoring closely. To ensure that there are sufficient resources to conduct planned, baseline inspections, the CNSC aims to recruit enough new inspectors to compensate for natural attrition rates, and to retain the qualified staff already in place.
Lastly, while inspections are important, they are not the only regulatory oversight tool available to the CNSC to assess licensee compliance. Throughout 2021, CNSC staff also continued to review annual compliance reports submitted by licensees and monitor reported events. Both activities can provide indicators of licensee performance, which can supplement inspection findings. In addition, licensee programs are reviewed and evaluated as part of the licence assessments. High-quality assessments help ensure that licensees have strong programs in place.
Considering the full suite of compliance activities, as well as the ability to prioritize the most risk-significant inspections, CNSC staff have concluded that the impact on licensee performance from the continued reduced number of inspections in 2021 remains acceptable. As restrictions continue to be lifted, CNSC staff anticipate that the number of onsite inspections will increase, which will in turn lead to increased confidence that nuclear substance licensees continue to operate in a safe manner. Staff presence at licensee sites will be a reminder to licensees that regulatory oversight and compliance with regulatory requirements continue to be priorities for the CNSC.
2.0 Inspection planning overview
Across Canada, there are over 2,000 nuclear substance licences issued by the CNSC and over 3,000 locations subject to CNSC inspections (since licensees can have more than one location). Given this high volume of licences and the finite number of inspectors, the Directorate of Nuclear Substance Regulation (DNSR) needs to deploy its resources to ensure maximum effectiveness. This is done through the application of a risk-informed inspection planning process, where resources and regulatory oversight are applied commensurate with the risk posed by the regulated activities.
The DNSR inspection planning process involves many considerations. First, risk rankings are established for each type of licensed activity, which provides a relative order of regulatory effort (in the areas of both licensing and compliance) to be applied to that activity. This risk ranking is the primary driver for setting a baseline inspection frequency for a specific facility or activity. The baseline is defined based on a well-performing licensee who meets all regulatory requirements in a particular area and is determined by CNSC staff using the risk index approach in accordance with CAN/CSA-IEC/ ISO 31010-10, Risk Management – Risk Assessment Techniques. Experience has demonstrated that not all licensees within a particular subsector require the same inspection frequency.
With this initial baseline established, staff prioritize inspections on a yearly basis using a risk-informed approach that takes into account specific criteria. The prioritization exercise may consider any or all of the following criteria:
- licensees most overdue for inspection
- new licensees (never inspected, potential unknown risks)
- compliance/performance history at the licensee level, including enforcement actions
- sector and/or subsector performance trends
- instigating factors (e.g., changes in radiation safety officers, amalgamation of licences, acquisitions, mergers and licence transfers)
- staff input based on knowledge of a particular licensee
- list of licensees or locations that require a follow-up inspection
The prioritization process considers all licensees that are due for an inspection according to the baseline. The process then involves assessing the risk profile of the licensees according to the frequency, recurrence and severity of past non-compliances; reviewing performance and compliance trends; and creating the priority lists based on defined criteria.
The priorities are separated into 3 categories:
- Priority 1 (P1) inspections must be performed as assigned, or declined with a defensible and documented rationale (i.e., decommissioned location, inaccessible, inventory not present).
- Priority 2 (P2) inspections, while not required to be performed, should be prioritized above all other remaining options.
- Priority 3 (P3) inspections are the remainder of the inspections that are due and can be performed after P1 and P2 inspections have been considered.
The prioritization of inspections into 3 categories helps ensure that staff are inspecting licensees and locations where the need is greatest. It allows flexibility to be built into the plan. The execution of the inspection plan is then dependent on available resources and on restrictions (e.g., travel constraints during the pandemic). It is also dependent on unplanned work that must be addressed. For example, approximately 30% of compliance effort is spent on reactive work related to the follow-up of non-compliances by licensees or to verify licensee response to events. The amount of effort expended on these activities will vary depending on the performance of licensees and on the number of events reported that require follow-up.
Overall, the inspection planning process is effective at ensuring that resources are applied appropriately for compliance oversight purposes. In light of the restrictions imposed by the pandemic, this established process allowed the CNSC to accommodate a reduction in annual inspections without compromising safety, by concentrating its resources on those highest priority inspections.
3.0 Compliance performance
Appendix B covers the full 2021 performance data, broken down by SCA, sector and subsector. In addition, the data shows the 5-year performance trends within each of these categories.
Overview of compliance framework
To measure licensee performance, CNSC staff use the well-established Safety and Control Area Framework. The framework includes 14 safety and control areas (SCAs) covering all technical areas of regulatory oversight. While CNSC staff review and assess performance in all SCAs (if applicable) as part of licensing and compliance activities, the ROR focuses on those that are most effective in providing an overall indication of the safety performance of the licensees, namely, the management system, operating performance, radiation protection, and security SCAs. Performance data in the environmental protection and the conventional health and safety SCAs is also provided for the waste nuclear substance licensees. These licensees, unlike other licensees covered by this report, have a higher potential for environmental releases. In addition, given the nature of the work performed and the introduction of other hazards to be mitigated, there is a potentially higher risk in conventional health and safety.
During licensing and compliance activities, CNSC staff evaluate the licensee’s performance within each relevant SCA by reviewing licensee documents and conducting inspections. Owing to the broad nature of the different activities conducted by the licensees covered, not all SCAs apply to all activities or all licensees. All relevant SCAs are assessed during inspections and a compliance rating is assigned for each one. Individual SCAs may include multiple assessment areas. The areas or items to be assessed arise from regulatory requirements, licence conditions, and documents referenced in the licence. Some of these requirements are administrative in nature and are considered relatively low risk, while others are linked to an immediate risk to health, safety or security, and therefore any findings against these items during an inspection must be addressed immediately. Refer to appendix H for an inspection worksheet template used for a typical inspection of nuclear substances and radiation devices licensees, specifically those in the portable gauge subsector. All areas assessed within a given SCA impact the overall rating of that SCA. A description of the ratings is provided in appendix I. The ratings definitions were updated in 2021 to improve clarity. CNSC staff presented the updated definitions and provided an explanation of the revisions to the Commission in January 2022 as part of the Update on the CNSC Staff Review of the Regulatory Oversight Report Process presentation.
CNSC staff track and follow up on all required corrective actions arising from less than satisfactory performance (“below expectations” or “unacceptable” ratings) to ensure that all items of non-compliance are addressed to their satisfaction. In the event an immediate risk to health, safety or security is identified, CNSC staff take prompt action, which may include enforcement actions such as the issuance of orders. Administrative monetary penalties (AMPs) may also be used as part of a graduated approach to compliance for issues that do not pose an immediate risk to health or safety.
Feedback from stakeholders: Additional information on SCAs in the 2021 ROR
CNSC staff acknowledge that all SCAs are important, regardless of whether they are included in the ROR. Based on interventions in previous ROR proceedings and on follow-up discussions with intervenors, the packaging and transport and the environmental protection SCAs continue to be the areas where stakeholder interest is high.
The interest in packaging and transport is not surprising given the volume of shipments of nuclear substances made each year. However, the packaging and transport SCA is not applied in the same manner across all licensees and depends on the nature of the industry. For example, the packaging and transport SCA is of high importance in the portable gauge or industrial radiography subsectors as these are mobile radiation devices that are transported daily. In comparison, within the fixed gauge subsector, the packaging and transport SCA is of minimal importance as these radiation devices are generally in a fixed place for lengthy periods of time and might only be packaged for transport at the beginning and the end of their life. Comparing data for these different subsectors becomes difficult as the activities performed are varied, and it would be challenging to present performance data in a meaningful way in the ROR. Instead, a review of the reported events related to packaging and transport provides a more meaningful indicator for this SCA across the sectors. As was done as part of the 2020 ROR, further analysis of this indicator is provided in appendix E.
With regard to the environmental protection SCA, much of the stakeholder interest is related to how REGDOC-2.9.1, Environmental Principles, Assessments and Protection Measures, applies to the licensees covered by this report. Environmental protection is considered as part of all licence application assessments and as part of compliance oversight. For some licensed activities where there are no interactions with or releases to the environment, no additional environmental protection measures are required. For all other licensed activities, REGDOC 2.9.1 is applied in a graded manner. In response to interest in this area, CNSC staff have included additional information on this topic in section 3.7.
Overall analysis of 2021 compliance results
A total of 5 unacceptable ratings, as defined by the CNSC, were issued in 2021. All 5 were issued to the industrial sector and all were issued in the SCAs covered by this report, as described in the sections below. CNSC staff issued orders in 3 of the 5 instances and took licensing action in the other 2. No unacceptable ratings were issued in the SCAs not covered in this report.
A list of inspections performed in 2021 is available in appendix F. Where items of non-compliance were identified, CNSC staff verified that licensees took appropriate corrective actions. Licensees promptly addressed any items of non-compliance that had immediate risks to health, safety or security.
Overall licensee performance in the 4 sectors (medical, industrial, academic and research, and commercial) has remained satisfactory and relatively stable over the past 5 years in all SCAs covered by this report; however, there was more significant variation at the subsector level, as described below. This decline in performance in certain areas was not surprising and, in fact, was predicted in the 2020 edition of the ROR on the use of nuclear substances in Canada. While a number of factors influence compliance, CNSC staff believe that there is a link between the decline in performance and the reduced number of inspections in recent years, as described in section 1.0. In addition, downward trends in compliance over the past year could reasonably be attributed to other influences, including the typical adjustment period that follows the introduction of new regulatory requirements (notably for the radiation protection SCA, as described below), as well as the impact of the pandemic on licensee operations, particularly in the medical sector. Lastly, it is always important to keep in mind that, for some sectors, overall performance in a given SCA is extrapolated based on very small datasets, which limits the reliability of any conclusions drawn.
Regardless of the reasons for the declining performance, CNSC staff continue to monitor compliance results and use those results as one of the inputs for the risk-informed approach to regulating these sectors. In addition, the CNSC maintains the capacity to respond quickly to an event and, as already mentioned, ensures that licensees promptly address any risk-significant areas of non-compliance.
While not showing a particular decline this past year, the 2021 performance results in certain SCAs for some sectors continued a trend of slightly poorer performance over the last 5 years. In particular, this applies to the radiation protection SCA in the medical sector and the operating performance SCA in the fixed gauge subsector. Although the majority of licensees consistently achieve satisfactory ratings in these SCAs, CNSC staff have developed and implemented various strategies in recent years to promote increased compliance. While, admittedly, more work needs to be done in these sectors, the CNSC’s current focus on regaining the baseline inspection frequency means that it is not actively developing new regulatory responses at present. Until that baseline has been regained, CNSC staff will continue to closely monitor compliance indicators in these areas and will maintain the capacity to address any serious concerns in an expedited manner.
When reviewing the compliance results for 2021, it is important to put them in context: while they represent a key indicator of licensee performance, they are not the only one used. In addition to analyzing compliance data, CNSC staff also make use of other indicators, including those reported on in this ROR, in assessing risk and making decisions about how to implement our regulatory oversight.
A brief overview of the SCAs is provided below, with more details provided in appendix B. Appendix B.5 presents the inspection results by subsector, offering another perspective on licensee performance in 2021.
3.1 Management system
The management system SCA covers the framework that establishes the processes and programs required to ensure that an organization achieves its safety objectives, continuously monitors its performance against those objectives, and fosters a healthy safety culture.
In 2021, all sectors performed well in this SCA, with 97% of inspections receiving satisfactory ratings. This is comparable to previous years. There were no unacceptable ratings in this SCA.
Refer to appendix B.1 for additional information.
3.2 Operating performance
The operating performance SCA refers to the licensee’s ability to perform licensed activities in accordance with pertinent operational and safety requirements defined in the Nuclear Safety and Control Act (NSCA), its associated regulations, and licence conditions. Licensees are expected to demonstrate that they comply with operational and safety requirements by providing workers with appropriate procedures for the safe use of nuclear substances and prescribed equipment, by ensuring that workers follow procedures, and by maintaining records that demonstrate compliance.
In 2021, overall licensee performance in this SCA improved, with 87% of inspections yielding satisfactory ratings compared to 83% in 2020. Performance increased in all sectors except the commercial sector, where the percentage of inspections yielding satisfactory ratings in this SCA decreased by 7 percentage points compared to 2020.
One licensee in the industrial sector received an unacceptable rating in this SCA. As a note, the same licensee was issued an unacceptable rating in the security SCA. In response to these poor ratings, the CNSC issued order 1340 to the licensee. The licensee complied with all terms of the order to the satisfaction of the CNSC and the order is now closed.
Refer to appendix B.2 for additional information.
3.3 Radiation protection
Radiation protection programs are required for every licensee to ensure that contamination levels and radiation doses received by workers are monitored, controlled, maintained below regulatory dose limits, and kept at levels that are as low as reasonably achievable (ALARA), with social and economic factors being taken into account. Licensees are expected to monitor worker doses, post radiation warning signs, plan appropriately for radiological emergencies, manage oversight of operational activities, institute effective workplace practices that emphasize the use of time, distance and shielding to minimize exposure to radiation, and use appropriate protective equipment.
Overall, in 2021, licensees maintained similar ratings compared to previous years, with 83% of inspections resulting in satisfactory ratings in the radiation protection SCA. While the medical and industrial sector ratings were similar to previous years, both the academic and research sector and the commercial sector demonstrated a decline in performance. In particular, the academic and research sector showed a significant decline in performance, with only 78% of 23 inspections performed yielding a satisfactory rating in the radiation protection SCA in 2021 compared to 100% of the 10 inspections performed in 2020. As noted earlier, these performance results are an indicator that CNSC staff factor into their inspection planning. However, while 5 of the 23 inspections performed received a below expectations rating in 2021, it represents only a small proportion of the 187 licences in the sector, making it difficult to confirm whether this is representative of the entire sector.
The revised Radiation Protection Regulations came into effect in late 2020. Part of the regulatory oversight program involved having inspectors promote compliance with the amended regulations while performing compliance activities. As of mid-2021, all licensees were expected to have implemented all changes related to the amended regulations. It is therefore reasonable to conclude that this is another reason for declining performance, as licensees adjust to the new requirements. Notices of non-compliance were issued in cases where it was found that licensees had not updated their programs to align with the amended regulations.
There were 3 unacceptable ratings issued in the radiation protection SCA in 2021. All 3 were issued in the industrial sector. In one case, an order was issued (order 1223) to an industrial radiography licensee in response to this rating. The licensee complied with all terms of the order to the satisfaction of the CNSC and the order is now closed.
In the second case, the licensee agreed to put all its portable gauges in storage and requested an amendment to its licence to reflect this change until a new radiation safety officer is in place and its radiation protection program is adequately implemented. As of the writing of this report, all portable gauges remain in storage.
The last unacceptable rating in this SCA was issued to a fixed gauge licensee. It was determined that there had been a significant failure by the licensee to implement its radiation protection program and that there was a lack of management oversight of the licensed activities. The licensee was intending to request the revocation of its licence and had transferred 4 of its 5 fixed gauges for disposal just prior to the inspection. However, one of the gauges (a low risk, category 4 sealed source) that had been in storage was lost (event ID 5357) and has not been found as of the writing of this report. The licence was revoked in May 2022; however, the licensee has committed to continuing the search and has a plan in place for if and when it is found. This plan includes immediately notifying the CNSC if the gauge is found and arranging for its disposal, including covering the costs.
Refer to appendix B.3 for additional information.
3.4 Security
Licensees are required to have in place physical security measures, practices and programs to prevent the loss, illegal use, illegal possession or illegal removal of nuclear substances during their entire lifecycle, including while they are in storage or during transport, as per the NSCA. The extent of the security measures required depends on the types of nuclear substances used and activities performed by each licensee.
In 2021, nuclear substance licensees maintained strong compliance with applicable security requirements, including the general requirements contained in regulations and in REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources and Category I, II and III Nuclear Material, applicable to sealed sources and radiation devices. Consistent with previous years, 91% of licensees inspected received a satisfactory grade in this SCA.
However, the medical sector demonstrated a drop in performance, with only 79% of the 34 inspections receiving a satisfactory grade in 2021 compared to 97% of the 33 inspections performed in 2020. In this sector, it is important to note that only 7 inspected licences (of the 440 medical sector licences) received a below expectations rating; therefore, the data may not be sufficient to conclude that there has been an overall decrease in performance for the whole sector. Furthermore, the projected link between a reduction in inspections and a decline in licensee performance is particularly relevant for the security SCA, since certain elements of security inspections cannot be performed remotely.
There was also a significant increase in the number of security-related events reported in 2021(more than double the average of the previous 4 years), with over 40% reported in the medical sector. The actual risk presented by these security breaches was low, and no nuclear substances were lost as a result of any of the events. This is further discussed in section 6.0.
As mentioned earlier, one licensee in the industrial sector received an unacceptable rating in both the security SCA and the operating performance SCA. In response to these poor ratings, order 1340 was issued to the licensee and is now closed.
Refer to appendix B.4 for additional information.
3.5 Conventional health and safety for waste nuclear substance licensees
The CNSC requires WNSLs to have a program in place to manage workplace safety hazards and to protect workers given the nature of the work and the introduction of other hazards that need to be mitigated. For example, WNSLs handle, process, store and transport different types of radioactive waste, which may require the use of overhead cranes and large equipment. The licensed activities directly introduce mechanical, ergonomic, chemical, electrical and fire hazards that need to be mitigated.
In 2021, no WNSLs received below expectations or unacceptable ratings in the conventional health and safety SCA.
The licensees continued to implement health and safety programs in accordance with the applicable occupational health and safety legislation to protect the health and safety of their workers.
3.6 Environmental protection for waste nuclear substance licensees
WNSLs are required to have specific programs in place to identify, control and monitor all releases of radioactive and hazardous substances and their effects on the environment.
In 2021, no WNSLs received below expectations or unacceptable ratings in the environmental protection SCA. The WNSLs continued to manage and monitor environmental releases relating to licensed activities.
WNSLs reported 3 events that could potentially have impacted the environment in 2021.
In the first event, the licensee reported that a holding tank of water from laundry was inadvertently discharged to the sewer. The intent was to transfer the water to a different tank, but the operator missed that the drain valve to the sewer was open. The water had been sampled beforehand, and results indicated that radiological parameters were all well below derived release limits and action levels. However, the concentrations of total suspended solids, total phosphorous (non-radioactive) and biological oxygen demand exceeded the municipality’s by-law limits. The municipality confirmed that there were no adverse conditions detected in the municipal sewage treatment plant as a result of the discharge.
In the second reported event, consolidated plant wastewater streams were discharged from a holding tank with the appropriate approvals as specified by procedures. A consolidated sample was preserved and subsequently analyzed, and the total non-radioactive phosphorous concentration indicated that there was a minor exceedance of 0.75 ppm over the limit (limit is 10 mg/L). Prior to the transfer to the holding tank, a separate analysis of the waste streams indicated that the consolidated liquid would be below the limit. The licensee believes there was an issue related to recirculation in the holding tank, resulting in a non-homogeneous sample being tested. There was no requirement to immediately notify the municipality, and it was reported in the licensee’s quarterly report submission to the municipality. There were no adverse environmental effects.
Lastly, one licensee reported that it had been sampling local air and not the air released from the stacks for a period of a few weeks to a month due to equipment failures. No work with potential for high levels of airborne tritium was conducted in the area that the stack in question serves. Based on the work performed during that period, an estimated 2 GBq/week of tritium was emitted from the stack, which is well below the action level, and there was no work conducted in this period that could have produced higher results. All other parameters, both radiological and non-radiological, were below the relevant limits and action levels.
All releases were kept well below regulatory limits, and there was no impact on the health and safety of persons and the environment.
3.7 Environmental protection for other nuclear substance licensees
Performance results in the environmental protection SCA for nuclear substance licensees are not typically included in the ROR, since those licensees have minimal to no interactions with the environment. In response to questions raised by intervenors on previous RORs, CNSC staff are providing a more fulsome explanation of the rationale for excluding this SCA from the report.
CNSC staff apply REGDOC-2.9.1, Environmental Principles, Assessments and Protection Measures, when reviewing applications for nuclear substance licences to ensure that there are no significant interactions with the environment. These licences generally cover activities involving the use of sealed or unsealed sources.
For sealed sources (e.g., fixed or portable gauges used in the industrial sector that contain sealed sources), the analysis performed led staff to conclude that there are no routine interactions with the environment and that, therefore, there is no need for an environmental risk assessment and little to assess under the environmental protection SCA.
For unsealed sources (e.g., radioisotopes used in the medical sector), there is a higher chance of interaction with the environment. This could involve, for example, releases from the stacks of isotope processing facilities or the disposal of small amounts of medical isotopes to the landfill or the sewer. For these licensees, CNSC staff apply the requirements in REGDOC-2.9.1, Environmental Principles, Assessments and Protection Measures, in a graded manner. Additional information on the application of REGDOC-2.9.1 to the nuclear substance licensees covered in this ROR is summarized below and will be covered in the proposed draft REGDOC 2.9.2, Controlling Releases to the Environment, which is currently under development and is scheduled to be presented to the Commission in September 2022.
In general, for licences authorizing the use of unsealed nuclear substances, the following apply with respect to disposal or releases:
- standard exemption quantity (EQ) and unconditional clearance levels specified in Schedules 1 and 2, respectively, of the Nuclear Substances and Radiation Devices Regulations
- generic conditional clearance levels (CCLs) documented in REGDOC 2.9.2, Controlling Releases to the Environment, provided that releases occur only through the specified pathway (i.e., solids to municipal landfill, gases to atmosphere, liquid to municipal sewer)
- practice-specific conditional clearances, which are CCLs applicable only to a defined practice or activity and which have been developed by the CNSC for application to multiple licensees carrying out the specific practice or activity; they are generally included as a condition on a licence
The majority of licensees covered by this ROR do not use unsealed sources as part of their licensed activities. Therefore, reporting on this SCA would not be effective in providing an overall indication of the safety performance of the licensees covered by this report. Licensees must, however, have programs in place to ensure that they meet any release limits imposed on them, and program implementation is verified during inspections and desktop reviews.
CNSC staff conclude that the oversight of the environmental protection SCA for nuclear substance licensees and the assessment and controls put in place for environmental protection for the industry are such that there is no additional benefit to highlighting performance in this SCA for all licensees covered in this report.
4.0 Enforcement
Appendix C presents enforcement action data by sector over the past 5 years and includes a list of all orders and AMPs issued in 2021.
The CNSC uses a graduated approach to enforcement in order to encourage compliance. When non-compliance (or continued non-compliance) has been identified, CNSC staff assess the significance of the non-compliance and determine the appropriate enforcement action, including but not limited to orders and administrative monetary penalties (AMPs).
In 2021, CNSC staff issued 10 orders and 1 AMP. All enforcement actions were issued to licensees in the industrial sector, with the exception of 1 order that was issued to a non-licensee in possession of radiation devices without a licence authorizing such possession. The fact that all but 1 enforcement actions were issued in the industrial sector is consistent with trends from previous years.
As expected, since enforcement actions tend to be issued as a result of findings during inspections, the number of enforcement actions issued in 2021 was higher than the number issued in 2020, given that the number of inspections performed also increased. Although neither the number of inspections nor the number of enforcement actions have reached pre-pandemic levels, staff anticipate that as the inspection numbers increase moving forward, the number of enforcement actions will also increase. All enforcement actions issued in 2021 are closed, and the CNSC is satisfied that the licensees and the 1 non-licensee have addressed the conditions of the orders/AMPs.
Enforcement actions are posted on the CNSC regulatory actions web page as they are issued.
5.0 Effective doses to workers
Appendix D presents the full datasets, as well as additional information, on effective doses to workers in 2021
Licensees are required to keep radiation doses to persons below regulatory limits and as low as reasonably achievable (ALARA) in accordance with the radiation protection program referenced in their licence.
Licensees must report the doses to their workers, whether estimated or measured, as part of their annual compliance reports. In 2021, doses were reported for 56,040 workers in the 4 sectors. Of those workers, 24,066 were nuclear energy workers (NEWs). The remaining 31,974 were not identified as NEWs and are referred to as non-NEWs in this report. Exposures to radiation continued to be very low for workers covered in this ROR for 2021, consistent with previous reporting years.
In 2021, no NEWs received doses above the regulatory limit of 50 mSv per calendar year. Of the 31,974 non-NEWs for which doses were reported, there were 7 reported doses greater than the regulatory limit of 1 mSv/year.
In one case, a portable gauge licensee reported that a seasonal worker had received a dose of 3.82 mSv. Based on the work performed by the worker, the dose was deemed non-personal and likely resulted from improper storage of the dosimeter. The licensee began the process for a dose change request, but was not able to reach the worker to have him sign it as he was out of the country. The licensee subsequently received an email resignation from the employee. The licensee tried again to reach him but was unsuccessful; therefore, the dose change request will not proceed.
Five of the doses that were reported as being above the 1 mSv/year limit for non-NEWs involved 2 licensees who did not correctly identify workers likely to exceed the regulatory limit. All 5 of the non-NEWs worked in the portable gauge subsector (2 for one licensee and 3 for another). Their doses were estimated based on the number of “shots” using a portable gauge. One shot is equal to approximately 1.2 mSv. The maximum number of shots by any of these workers was 1,962, which would be the equivalent of approximately 2.4 mSv. In all 5 cases, the workers left their employment before they could be acknowledged as NEWs. In these cases, it was the licensees that failed to correctly assess the work to be performed by their workers, identify those workers likely to exceed the 1 mSv dose limit, and acknowledge them as NEWs. CNSC staff consider this to be an administrative non-compliance.
Lastly, in one case, a worker in the academic and research sector received a dose of 1.3 mSv. The licensee investigated the dose and concluded that the worker did not follow established safe work practices, leading to the elevated dose. The licensee reviewed all its workers' doses for the past 5 years and confirmed that all laboratory personnel are typically well below 0.2 mSv. The licensee implemented corrective actions to prevent recurrence. This event was presented to the Commission as an event initial report (EIR) on January 21, 2021 (CMD 21-M10).
There was strong performance in the industry in 2021, with doses to all workers remaining generally low. Of the 7 reported instances where a worker not designated as a NEW exceeded the regulatory limit, only 1 was deemed as a true exceedance of the regulatory limits; no health effects are expected in that case.
6.0 Reportable events
Appendix E provides data on the types of events reported over 5 years and describes each event reported in 2021.
Licensees are required to have programs in place to manage unplanned events and accidents. The events that warrant mandatory reporting and the content of those reports are set out in the NSCA, its regulations and the licence conditions. CNSC staff review, assess and track all events reported by licensees.
Since 2014, reported events have been rated using the International Nuclear and Radiological Event Scale (INES), a 7-point scale for communicating the safety significance of nuclear and radiological events to the public. Note that the scale is not a tool for comparing safety performance among facilities or organizations, but rather, for effectively communicating the safety significance of events. CNSC staff assign a ranking to each event based on the INES scale.
The events reported to the CNSC by the licensees covered in this ROR typically fall into level 0 (no safety significance), 1 (an anomaly that may have an impact on defence in depth) or 2 (incident that may have more significant impacts on defence in depth, impacts on people or the environment, or impacts on radiological barriers and controls).
CNSC staff assessed 171 events related to nuclear substances and prescribed equipment in 2021. Of these events, 165 were rated as INES level 0, and 6 as INES level 1. Of those rated as INES level 1, 5 involved the theft of portable gauges and 1 involved the loss of a fixed gauge. All 6 events involved category-4 sealed sources (i.e., low risk). Of the 5 stolen portable gauges, 2 were recovered and returned to the owner, while the other 3 have not yet been recovered. The lost fixed gauge has not been found.
While the 171 events reported in 2021 represent an increase in comparison to the 135 reported in 2020, the number is more consistent with the years preceding 2020. In 2020, the numbers reported were likely lower due to initial lockdowns and/or licensee restrictions on their operations during the initial stages of the pandemic. Although lockdowns related to the pandemic occurred in 2021 as well, most licensees maintained normal or near normal operations, meaning that the number of events reported also returned to more normal levels.
Overall, the most significant change in the types of events reported was in the area of security events. Significantly more breaches of security were reported in 2021 compared to the previous 4 years, as seen in Appendix E, figure 18. Over 40% of the reported breaches of security were in the medical sector, with most of those reports involving unattended nuclear substances, unsecured doors, or disarmed alarm systems. As mentioned previously, medical sector licensees experienced significant impacts due to the pandemic that may have led to changes in staffing, day-to-day operations and overall workflow, any of which could have resulted in an increase in events. One medical sector licensee in particular reported multiple events. The licensee launched a review of its security program, and CNSC staff have a strategy in place to manage this particular licensee, including a focused security inspection in 2022. It is important to note that while the numbers trended upwards in 2021, the actual risk presented by these events was low and no nuclear substances were lost as a result of any of the events. Licensees employ multi-layered security programs. The failure of one or two barriers did not lead to the breakdown of other barriers, which remained intact until the incidents were discovered and corrective action was taken.
There was also an increase in the number of spill, contamination and release events reported in 2021 compared to previous years. Over 60% of these were reported by the nuclear medicine subsector and involved spills or contamination with short-lived radioisotopes. None of the 26 events reported resulted in impacts to the environment. Although none of the events resulted in any exposures above the regulatory limit and all were rated as INES level 0, a significant event was reported by a licensee in the commercial sector that led to an unplanned exposure to a NEW. A spill of greater than 100 EQ of iodine-131 occurred in a radiation-shielded box, under a vented hood. The subsequent clean-up resulted in an unplanned exposure to a NEW of 29 mSv (effective dose) and a committed equivalent dose to the thyroid of 560 mSv, both of which are below regulatory limits. The unplanned dose was determined to be a result of a gap in licensee procedures and a failure of the worker to follow thyroid monitoring procedures. The licensee has taken appropriate corrective measures to address these issues and the CNSC has shared lessons learned with other similar licensees. A verbal EIR was presented to the Commission in April 2021, with subsequent follow ups in October 2021 and March 2022.
For all events reported to the CNSC, licensees implemented appropriate response measures to mitigate the impacts and to limit radiation exposure to workers and the public. CNSC staff reviewed the measures and found them to be satisfactory.
6.1 Update on Mississauga Metals & Alloys Inc.
In the first quarter of 2021, Mississauga Metals & Alloys, Inc. (MMA), one of the 5 waste nuclear substance licensees (WNSLs) covered by this ROR, applied for and received a short-term renewal of its waste nuclear substance licence. The licence included a condition that MMA would comply with an arrears payment schedule as set out in its licence. On August 20, 2021, MMA declared bankruptcy and CNSC staff undertook numerous actions as a result. An EIR was presented to the Commission on October 5, 2021, and an update was provided on November 23, 2021, during the presentation of the Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2020.
Since then, CNSC staff have continued to undertake 3 main activities related to this complex file:
- ensuring that the site remains safe and secure, and adapting the approach taken as site conditions change
- pursuing the provision of a contract to a third-party expert to undertake the detailed characterization of the waste
- working with other interested parties (e.g., bankruptcy trustee, key creditors, other levels of government) to find a solution regarding authority to undertake the needed work on the site (e.g., characterization, disposition)
The waste nuclear substance licence expired on February 28, 2022 and has not been renewed. The CNSC continues to ensure the safety and security of the nuclear substances on the site.
6.2 Update on the fatality at Kinectrics Inc.
On June 14, 2021, a contractor was providing services to Kinectrics Inc., another WNSL, when an industrial accident led to the fatality of one of the contractor’s employees. The Ministry of Labour, Training and Skills Development for the Province of Ontario was notified, and it began an investigation on June 14, 2021, which is expected to conclude in June 2022. An EIR was provided to the Commission at the October 5, 2021, meeting and CNSC staff will provide a further update during the presentation of this ROR in November 2022.
7.0 Public and stakeholder engagement
Appendix G includes a complete list of engagement activities undertaken in 2021.
The CNSC carries out stakeholder engagement and outreach activities to facilitate communication between the CNSC and the nuclear substance licensees and other stakeholders on licensed activities and regulatory expectations. To date, Indigenous Nations and communities have not expressed a specific interest in this ROR or in the licensed activities that it covers. However, upon request, CNSC staff have participated in general outreach activities with Indigenous Nations and communities to provide information on the packaging and transport of nuclear substances, but not specifically related to the licensees covered by this report. CNSC staff remain open and committed to ongoing engagement and communication with any interested Indigenous Nations and communities who may express an interest in discussing the topics and licenses covered in this ROR.
Stakeholder engagement and outreach are critical elements of the CNSC’s regulatory approach. Given the breadth of licensees regulated in the area of nuclear substances, a particular focus is on reaching and engaging with licensee communities, a practice that leads to increased awareness and better understanding of the regulatory process and requirements. CNSC staff leverage a variety of fora to engage with licensees and promote the use of the tools that are developed to support compliance with regulatory expectations.
In 2021, all outreach was done virtually or through written communications. Outreach included participation in town hall sessions, regular publication of the DNSR Digest, emails to targeted groups of licensees, meetings with associations or working groups, presentations at industry conferences, and the publishing of articles in industry publications. For a complete list of outreach activities, refer to appendix G.
8.0 International obligations and commitments
The mandate of the CNSC includes adherence to international commitments to which Canada is a party. CNSC staff had removed the section describing those commitments from previous RORs in the interest of streamlining the report. However, based on recent feedback from intervenors, this section has been reinserted.
Canada has committed to the implementation of various International Atomic Energy Agency (IAEA) codes, standards and guidance documents. For example, as part of Canada’s commitment to the IAEA Code of Conduct on the Safety and Security of Radioactive Sources, nuclear substance licensees with Category 1 and/or 2 (high-risk) sealed sources must inform the CNSC of any transfer, receipt, export or import of those sources. Licensees are to report their high-risk sealed sources inventory through the Sealed Source Tracking System (SSTS). The SSTS is a secure information management system that tracks new and existing high-risk sources within Canada. It populates the National Sealed Source Registry so that the information is as current as licence reporting allows. Licensees subject to this requirement have the relevant licence condition included on their licence, and compliance with this condition is verified through a regulatory inspection.
In addition, the CNSC considers international regulations and standards when developing domestic regulations. For example, the Packaging and Transport of Nuclear Substances Regulations, 2015, with which all licensees and non-licensees must comply, incorporate by reference the IAEA Regulations for the Safe Transport of Radioactive Material.
The Government of Canada also has obligations on the peaceful uses of nuclear energy pursuant to the Treaty on the Non-proliferation of Nuclear Weapons. CNSC requirements for nuclear substance licensees relating to Canada’s international obligations are defined in the applicable regulations and licences.
Safeguards is a system of inspection and other verification activities undertaken by the IAEA to evaluate Canada’s compliance with its obligations pursuant to its safeguards agreements with the IAEA. The objective of the Canada–IAEA safeguards agreements is for the IAEA to provide assurance to Canada and to the international community that all declared nuclear materials are in peaceful, non-explosive uses and that there is no indication of undeclared nuclear materials or activities. The CNSC has published REGDOC-2.13.1, Safeguards and Nuclear Material Accountancy, which sets out the requirements and guidance for the establishment and maintenance of a safeguards program. Safeguarded materials include uranium, thorium and plutonium-239. Generally, among the licensees covered in this report, this material can be present as samples, check sources and shielding, among other forms. Licensees subject to safeguards have a condition included on their licence, and the CNSC continues to engage with licensees to ensure that all nuclear material subject to safeguards is reported to the IAEA. In 2021, the IAEA performed 3 inspections and 4 complementary accesses at the facilities of nuclear substance licensees to confirm licensees’ declarations on the possession and use of nuclear material. While the IAEA reported that the results from these inspections were satisfactory and that its inspectors were able to carry out all planned activities for the complementary accesses, it identified follow-up actions for the licensees and/or the CNSC. The CNSC is coordinating the resolution of these items with the licensees.
Nuclear substance licensees who import or export nuclear substances are subject to licence conditions that limit the types and amounts of nuclear substances that can be imported or exported by the licensee without a separate, valid import/export licence. In addition, REGDOC-2.13.2, Import and Export, sets out guidance for current and prospective licensees who intend to import or export risk-significant radioactive sources (Category 1 and 2 radioactive sources). Compliance with import and export restrictions are verified as a part of standard regulatory oversight.
CNSC staff ensure that licensees implement the measures required to meet Canada’s international obligations and commitments.
9.0 Conclusion
In 2021, most inspected licensees were in compliance with regulatory requirements and achieved satisfactory ratings in the SCAs reported on in this report. Licensing and certification activities continued to play a critical role in ensuring that licensee programs were in place. Strong programs contributed to overall licensee performance. Where compliance did not meet expectations, licensees implemented appropriate corrective actions. All enforcement actions in 2021 have been closed. Radiation exposure to workers continued to be very low and was consistent with previous years. When events did occur, licensees took appropriate measures to address the events and took steps to prevent recurrence.
Regulatory oversight of licensees in 2021 was again impacted by the pandemic; however, a gradual return to in-person inspections, along with the use of hybrid and remote inspections, allowed for a more holistic inspection experience. After gaining experience with remote inspections in 2020, CNSC staff were able to pivot between remote, hybrid and in-person inspections, as the pandemic allowed, in 2021. Staff continue to address the backlog of inspections and will continue to monitor for possible negative trends in compliance over the coming years.
Overall, CNSC staff saw no significant changes in licence distribution, compliance trending, or doses to workers for any of the sectors covered by this report. Licensees corrected identified items of non-compliance to the satisfaction of CNSC staff. The evaluations of findings for the SCAs, resulting from the CNSC’s comprehensive regulatory oversight of the industry, demonstrate that licensees made acceptable provisions to protect health, safety, security and the environment from the use of nuclear substances and prescribed equipment, and implemented the measures required to meet Canada’s international obligations. Based on these evaluations, CNSC staff conclude that the use of nuclear substances and prescribed equipment in Canada remains safe and secure.
Appendix A: Regulatory program for the use of nuclear substances
This appendix presents additional regulatory data to complement the information provided in the main part of the document.
A.1 CNSC regulatory effort
CNSC designated officers made a total of 2,085 licensing and certification decisions related to activities covered in this report in 2021. The majority of these were licensing decisions, as shown in table 1. There was no significant change in the number or type of decisions made compared to 2020.
| Type of decision | Number of decisions |
|---|---|
| Licensing (issuance of new licences, licence renewals, licence amendments, licence revocations and licence transfers) | 1,621 |
| Certification of prescribed equipment (radiation devices, Class II prescribed equipment, and transport packages) | 70 |
| Certification of exposure device operators (EDOs) (issuance of new certifications and renewal of certifications) | 379 |
| Certification of Class II radiation safety officers (RSOs) | 15 |
| Total | 2,085 |
The CNSC’s risk-informed regulatory program applies resources and regulatory oversight commensurate with the risk associated with the regulated activity. Regulatory effort related to licensing, certification and compliance verification is derived from this program. As shown in table 2, CNSC staff’s direct effort for regulating the use of nuclear substances in 2021 amounted to 12,551 person days or the annual equivalent of approximately 57 full-time staff. This is slightly higher than the 11,698 person days (53 full-time staff) directed to this program in 2020.
| Activity | Person-days | Full-time equivalents |
|---|---|---|
| Licensing | 5,569 | 25.3 |
| Certification | 1,632 | 7.4 |
| Compliance verification | 5,350 | 24.3 |
A.2 Licensing
CNSC staff perform risk-informed technical assessments of applications submitted to the CNSC. The CNSC has produced a series of licence application guides to ensure that its expectations for applicants are clear, and to facilitate applicants’ interactions with the regulator.
In 2021, there were 2,097 nuclear substances and prescribed equipment licences held by 1,500 licensees in Canada (table 3). These licences were issued to entities throughout Canada, as shown in figure 1. In addition, 49 of these licences were issued to companies headquartered in other countries (primarily the United States). Many of these companies service prescribed equipment located in Canada, while others have operational facilities in Canada.
The disparity between the number of licences and the number of licensees can be explained by the fact that while most licensees perform a single licensed activity and therefore require only one CNSC licence, others perform varied activities that require a licence for each one. For example, a hospital may have multiple licences to cover radiation therapy facilities, diagnostic nuclear medicine, therapeutic nuclear medicine, nuclear substance processing, and research labs, each of which is covered by its own licence given the unique requirements and programs. CNSC staff work with these licensees to ensure that an appropriate level of regulatory control is maintained, while minimizing administrative burden wherever possible.
| Sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 457 | 436 | 438 | 445 | 440 |
| Industrial | 1,287 | 1,259 | 1,228 | 1,207 | 1,221 |
| Academic and research | 195 | 192 | 187 | 189 | 187 |
| Commercial | 252 | 248 | 237 | 238 | 249 |
| Total | 2,191 | 2,135 | 2,090 | 2,079 | 2,097 |
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 1: Text version
The map shows the distribution of licences by sector across Canada.
Yukon: 4 industrial licences
Northwest Territories: 3 industrial licences
Nunavut: 0 licences
British Columbia: 174 industrial licences, 18 academic and research licences, 44 medical licences, 16 commercial licences (total of 252 licences)
Alberta: 303 industrial licences, 11 academic and research licences, 39 medical licences, 32 commercial licences (total of 385 licences)
Saskatchewan: 32 industrial licences, 5 academic and research licences, 11 medical licences, 2 commercial licences (total of 50 licences)
Manitoba: 27 industrial licences, 8 academic and research licences, 13 medical licences, 4 commercial licences (total of 52 licences)
Ontario: 382 industrial licences, 80 academic and research licences, 188 medical licences, 115 commercial licences (total of 765 licences)
Quebec: 207 industrial licences, 56 academic and research licences, 118 medical licences, 37 commercial licences (total of 418 licences)
New Brunswick: 28 industrial licences, 4 academic and research licences, 14 medical licences, 3 commercial licences (total of 49 licences)
Prince Edward Island: 2 industrial licences, 1 academic and research licence, 3 medical licences, 0 commercial licences (total of 6 licences)
Nova Scotia: 24 industrial licences, 3 academic and research licences, 5 medical licences, 4 commercial licences (total of 36 licences)
Newfoundland and Labrador: 20 industrial licences, 1 academic and research licence, 5 medical licences, 2 commercial licences (total of 28 licences)
A.3 Certification of prescribed equipment
Certification of prescribed equipment confirms that the prescribed equipment is safe to use; that adequate measures are in place to protect the environment, the health, safety and security of persons, and national security; and that the design meets international requirements. Prescribed equipment includes radiation devices, Class II prescribed equipment, and transport packages, and requirements for certification are set out in the regulations. As seen in table 1, designated officers made 70 decisions related to the certification of prescribed equipment in 2021, an increase over 2020 when 63 decisions were made. Similar to licensing, CNSC staff perform risk-informed technical assessments of certification applications submitted to the CNSC. The CNSC has regulatory documents in place to ensure that its expectations for applicants are clear. Service standards for the certification of Class II prescribed equipment, radiation devices, and transport packages were formalized in 2021 in response to the implementation of the Service Fees Act. Information on the service standards and CNSC performance can be found on the CNSC website.
A.4 Certification of exposure device operators
Licensees are required under the Nuclear Substances and Radiation Devices Regulations to permit only CNSC-certified personnel and supervised trainees to use exposure devices containing nuclear substances. In 2021, the CNSC certified 61 new exposure device operators (EDOs) and renewed the certifications of 318 others, which is very similar to 2020 when the CNSC certified 61 new EDOs and renewed 332 certifications. In 2021, CSA PCP-09, Exposure Device Operator Personnel Certification Guide, was updated. The updated version clarifies existing guidance and introduces new guidance to address common issues faced by applicants, employers and training providers.
A.5 Certification of Class II radiation safety officers
All licensees that operate Class II nuclear facilities or that service Class II prescribed equipment must have a certified radiation safety officer (RSO) and a qualified temporary replacement. The RSO ensures that licensed activities are conducted safely and that all regulatory expectations are met.
In 2021, the CNSC certified 15 Class II RSOs, compared to 24 certifications in 2020. As in 2020, there were no Class II RSOs decertified in 2021.
Appendix B: Compliance performance
This appendix provides details regarding compliance in the 4 SCAs determined to be the most relevant in providing an overall indication of the safety performance of licensees in 2021.
It is important to note that a below expectations rating does not necessarily mean that a licensee’s actions were unsafe. It could mean any of the following: licensee performance does not meet CNSC staff expectations, the licensee has risk-significant non-compliance(s) or performance issue(s), and/or non-compliances or performance issues are not being adequately corrected. Staff will issue unacceptable ratings in cases where licensee actions are unsafe – in 2021, only 5 unacceptable ratings were issued.
In all cases, for any below expectations ratings, CNSC staff ensured that licensees took appropriate corrective actions. For all unacceptable ratings, CNSC staff issued orders or took licensing action, with restrictions lifted only once the CNSC was satisfied that all conditions were addressed by the licensee.
B.1 Management system
Of the 489 inspections that looked at the management system SCA, 97% of the licensees inspected demonstrated that adequate processes and programs were in place to achieve their safety objectives and received satisfactory ratings (figures 2 and 3).
There were no unacceptable ratings in this SCA.
.jpg/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 2: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Satisfactory | 840 (97%) | 843 (97%) | 739 (97%) | 321 (96%) | 472 (97%) |
| Below expectations | 23 (3%) | 29 (3%) | 22 (3%) | 14 (4%) | 17 (3%) |
| Unacceptable | 1 (0.2%) | 0 (0%) | 3 (0.4%) | 0 (0%) | 0 (0%) |
| Total number of inspections | 864 | 872 | 764 | 335 | 489 |
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 3: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 106 (96%) | 110 (94%) | 155 (95%) | 44 (92%) | 101 (96%) |
| Industrial | 605 (98%) | 608 (97%) | 475 (98%) | 254 (96%) | 329 (97%) |
| Academic and research | 71 (97%) | 85 (99%) | 73 (99%) | 9 (100%) | 18 (100%) |
| Commercial | 58 (94%) | 40 (98%) | 36 (97%) | 14 (100%) | 24 (92%) |
| All sectors combined | 840 (97%) | 843 (99%) | 739 (97%) | 321 (97%) | 472 (97%) |
B.2 Operating performance
For the operating performance SCA, 87% of the licensees inspected ensured that adequate processes and programs were in place to achieve their safety objectives (figures 4 and 5) and received satisfactory ratings. Staff performed 531 inspections of this SCA.
There was 1 unacceptable rating issued to an industrial sector licensee in this SCA. Details on this unacceptable rating can be found in section 3.2 of this report.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 4: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Satisfactory | 747 (85%) | 747 (84%) | 672 (86%) | 292(83%) | 463 (87%) |
| Below expectations | 128 (15%) | 144 (16%) | 110 (14%) | 61 (17%) | 67 (13%) |
| Unacceptable | 8 (1%) | 4 (0.4%) | 2 (0.3%) | 0 (0%) | 1 (0.2%) |
| Total number of inspections | 883 | 895 | 784 | 353 | 531 |
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 5: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 100 (86%) | 96 (77%) | 155 (88%) | 41 (80%) | 97 (84%) |
| Industrial | 511 (82%) | 528 (83%) | 409 (85%) | 225 (84%) | 318 (88%) |
| Academic and research | 73 (97%) | 79 (88%) | 70 (95%) | 9 (90%) | 22 (96%) |
| Commercial | 63 (94%) | 44 (92%) | 38(89%) | 17 (94%) | 26 (87%) |
| All sectors combined | 747 (85%) | 747 (84%) | 672 (86%) | 292 (83%) | 463 (87%) |
B.3 Radiation protection
In 2021, 561 inspections were conducted to evaluate compliance with the radiation protection SCA. Of the licensees inspected, 83% ensured that adequate processes and programs were in place to achieve their safety objectives and received satisfactory ratings (figures 6 and 7).
Three industrial sector licensees received an unacceptable rating in this SCA. Details on these unacceptable ratings can be found in section 3.3 of this report.
.jpg/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 6: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Satisfactory | 744 (85%) | 748 (84%) | 629 (80%) | 299 (84%) | 465 (83%) |
| Below expectations | 129 (15%) | 137 (15%) | 160 (20%) | 55 (16%) | 93 (17%) |
| Unacceptable | 3 (0.3%) | 6 (0.7%) | 1 (0.1%) | 1 (0.3%) | 3 (0.5%) |
| Total number of inspections | 876 | 891 | 790 | 355 | 561 |
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 7: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 94 (81%) | 95 (77%) | 132 (74%) | 42 (82%) | 113 (78%) |
| Industrial | 518 (84%) | 539 (85%) | 387 (80%) | 227 (85%) | 308 (85%) |
| Academic and research | 69 (93%) | 68 (77%) | 71 (96%) | 9 (90%) | 18 (78%) |
| Commercial | 63 (100%) | 46 (100%) | 39(81%) | 21 (100%) | 26 (90%) |
| All sectors combined | 744 (85%) | 748 (84%) | 629 (80%) | 299 (84%) | 465 (83%) |
B.4 Security
For the security SCA, 91% of the licensees inspected ensured that adequate processes and programs were in place to achieve their safety objectives and received satisfactory ratings (figures 8 and 9). Staff performed 228 inspections of this SCA.
One industrial sector licensee received an unacceptable rating for the security SCA. Details on this unacceptable rating can be found in section 3.4 of this report.
.jpg/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 8: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Satisfactory | 764 (90%) | 764 (92%) | 719 (95%) | 163 (93%) | 208 (91%) |
| Below expectations | 77 (9%) | 68 (8%) | 41 (5%) | 13 (7%) | 19 (8%) |
| Unacceptable | 9 (1.1%) | 1 (0.1%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Total number of inspections | 850 | 833 | 760 | 176 | 228 |
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 9: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 96 (81%) | 96 (91%) | 158 (94%) | 31 (94%) | 27 (79%) |
| Industrial | 552 (93%) | 587 (94%) | 457 (94%) | 116 (95%) | 155 (93%) |
| Academic and research | 66 (97%) | 57 (79%) | 72 (99%) | 7 (100%) | 10 (91%) |
| Commercial | 50 (94%) | 46 (93%) | 32 (91%) | 9 (90%) | 16 (100%) |
| All sectors combined | 764 (90%) | 786 (94%) | 719 (95%) | 163 (93%) | 208 (91%) |
B.5 Inspection ratings by sector
This section provides data at the sector and subsector level for each of the 4 key SCAs covered in this report. Any significant findings at the SCA level have been further explained in section 3 of this report, where additional analysis is included for the management system, operating performance, radiation protection and security SCAs. Given the small number of WNSLs, specific data related to the environmental protection and the conventional health and safety SCAs are not included in this section.
A breakdown by subsector is not provided for the security SCA, given the potentially sensitive information associated with that SCA.
B.5.1 Medical sector
Tables 4 to 7 show the inspection performance of licensees in the medical sector. Subsector performance is shown for the years 2017 to 2021 as a percentage of the inspections that received satisfactory ratings for the SCA. The total number of inspections conducted to assess performance in the SCA appears in parentheses. The number of inspections shown in the “Entire medical sector” row is the aggregate for the entire sector, including subsectors not highlighted.
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Management system | Nuclear medicine | 98% (91) | 96% (103) | 95% (103) | 94% (47) | 99% (89) |
| Management system | Radiation therapy | 82% (11) | 50% (6) | 100% (4) | 0% (1) | 70% (10) |
| Management system | Veterinary nuclear medicine | 100% (4) | 100% (4) | 75% (4) | (0) | 100% (3) |
| Management system | Entire medical sector | 97% (110) | 94% (117) | 95% (163) | 92% (48) | 96% (105) |
The reasons for below expectations ratings in the radiation therapy subsector varied from licensee to licensee but were most often related to repeated notices of non-compliance, procedures not being followed and poor management oversight of the program. CNSC staff continue to work with these licensees to correct items of non-compliance and to work on program deficiencies.
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Operating performance | Nuclear medicine | 86% (90) | 77% (104) | 87% (155) | 77% (48) | 83% (89) |
| Operating performance | Radiation therapy | 89% (18) | 67% (12) | 100% (21) | 100% (2) | 90% (10) |
| Operating performance | Veterinary nuclear medicine | 100% (4) | 100% (4) | 100% (3) | 100% (1) | 100% (3) |
| Operating performance | Entire medical sector | 87% (116) | 77% (124) | 88% (176) | 77% (51) | 84% (115) |
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Radiation protection | Nuclear medicine | 75% (89) | 74% (104) | 70% (155) | 73% (48) | 75% (119) |
| Radiation protection | Radiation therapy | 100% (19) | 100% (12) | 100% (13) | 100% (2) | 100% (20) |
| Radiation protection | Veterinary nuclear medicine | 100% (4) | 50% (4) | 100% (3) | 100% (1) | 33% (3) |
| Radiation protection | Entire medical sector | 81% (116) | 77% (124) | 74% (178) | 76% (51) | 78% (145) |
The nuclear medicine subsector continues to demonstrate lower performance in the radiation protection SCA. This finding has remained consistent over the last 5 years. The most frequent non-compliances have consistently been related to the lack of management oversight in the implementation of the radiation protection program. Items of non-compliance frequently include the failure of workers to conduct thyroid monitoring in accordance with licence conditions, the failure to demonstrate that sampling and counting methods meet licence criteria for detecting loose contamination, and the failure to calibrate survey meters at the required frequency. CNSC staff continue to work with these licensees to correct items of non-compliance and to work on program deficiencies. While staff are also continuing to prioritize inspections for medium-risk licensees, such as those in the nuclear medicine subsector, the focus is currently on those licensees that are overdue for inspections. There was also a drop in the performance of veterinary nuclear medicine licensees in this SCA; however, with the small number of inspections performed, it is difficult to say whether this is representative of the subsector.
| SCA | Sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Security | Medical sector | 81% (118) | 91% (119) | 94% (168) | 97% (33) | 79% (34) |
There has been a significant drop in medical sector licensee performance in the security SCA. Section 3.4 provides additional details on these findings.
B.5.2 Industrial sector
Tables 8 to 11 show the inspection performance of licensees in the industrial sector. Subsector performance is shown for the years 2017 to 2021 as a percentage of the inspections that received satisfactory ratings for the SCA. The total number of inspections conducted to assess performance in the SCA appears in parentheses. The number of inspections for the “Entire industrial sector” row is the aggregate for the entire sector, including subsectors not highlighted.
A breakdown by subsector is not provided for the security SCA, given the potentially sensitive information associated with that SCA.
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Management system | Portable gauge | 99% (303) | 98% (321) | 100% (215) | 98% (92) | 96% (171) |
| Management system | Fixed gauge | 94% (130) | 94% (112) | 94% (124) | 94% (94) | 98% (64) |
| Management system | Industrial radiography | 96% (136) | 96% (138) | 98% (114) | 98% (66) | 99% (82) |
| Management system | Oil-well logging | 100% (42) | 98% (43) | 100% (24) | 89% (9) | 93% (15) |
| Management system | Entire industrial sector | 98% (620) | 97% (608) | 98% (487) | 96% (261) | 97% (340) |
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Operating performance | Portable gauge | 82% (305) | 86% (326) | 82% (216) | 82% (98) | 89% (192) |
| Operating performance | Fixed gauge | 70% (136) | 68% (111) | 73% (124) | 71% (94) | 72% (64) |
| Operating performance | Industrial radiography | 89% (116) | 88% (138) | 93% (114) | 98% (66) | 95% (82) |
| Operating performance | Oil-well logging | 93% (42) | 86% (44) | 100% (24) | 100% (9) | 100% (14) |
| Operating performance | Entire industrial sector | 82% (625) | 83% (633) | 84% (484) | 82% (267) | 88% (363) |
The fixed gauge subsector continues to demonstrate low performance in the operating performance SCA. This finding has remained consistent over the last 5 years. The most frequent non-compliances have consistently included failure of workers to follow their obligations, failure to meet condition requirements related to vessel or hopper entry or related to the mounting and dismounting of gauges, and failure to maintain appropriate worker records. CNSC staff continue to work with these licensees to correct items of non-compliance and to work on program deficiencies. CNSC staff have also engaged with licensees through specific outreach activities related to vessel entry procedures, as that was one area of declining performance. While staff are continuing to prioritize inspections for medium-risk licensees, such as those in the fixed gauge subsector, the current focus is on those licensees that are overdue for an inspection.
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Radiation protection | Portable gauge | 82% (306) | 84% (326) | 74% (216) | 83% (98) | 81% (192) |
| Radiation protection | Fixed gauge | 80% (132) | 77% (111) | 73% (124) | 82% (94) | 80% (64) |
| Radiation protection | Industrial radiography | 90% (130) | 91% (138) | 92% (114) | 86% (66) | 93% (82) |
| Radiation protection | Oil-well logging | 86% (42) | 91% (44) | 92% (24) | 89% (9) | 93% (14) |
| Radiation protection | Entire industrial sector | 84% (620) | 85% (633) | 79% (483) | 84% (267) | 85% (364) |
| SCA | Sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Security | Industrial sector | 91% (610) | 94% (624) | 94% (484) | 92% (122) | 93% (167) |
B.5.3 Academic and research sector
Tables 12 to 15 show the inspection performance of licensees in the academic and research sector. Subsector performance is shown for the years 2017 to 2021 as a percentage of the inspections that received satisfactory ratings for the SCA. The total number of inspections conducted to assess performance in the SCA appears in parentheses. The number of inspections for the “Entire academic and research sector” row is the aggregate for the entire sector, including subsectors not highlighted.
A breakdown by subsector is not provided for the security SCA, given the potentially sensitive information associated with that SCA.
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Management system | Laboratory studies and consolidated use | 97% (73) | 99% (84) | 99% (74) | 100% (9) | 100% (16) |
| Management system | Entire academic and research sector | 97% (73) | 99% (86) | 99% (74) | 100% (9) | 100% (18) |
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Operating performance | Laboratory studies and consolidated use | 97% (74) | 88% (86) | 95% (74) | 89% (9) | 94% (16) |
| Operating performance | Entire academic and research sector | 97% (75) | 88% (90) | 95% (74) | 90% (10) | 96% (23) |
| SCA | Subsector / sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Radiation protection | Laboratory studies and consolidated use | 97% (74) | 88% (86) | 93% (74) | 100% (10) | 69% (16) |
| Radiation protection | Entire academic and research sector | 97% (75) | 88% (90) | 93% (74) | 100% (10) | 78% (23) |
There has been a significant drop in the performance of licensees in the radiation protection SCA in the laboratory studies and consolidated use subsector as well as in the entire sector overall. Additional details on these findings can be found in section 3.3 of the report.
| SCA | Sector | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Security | Academic and research sector | 96% (69) | 79% (72) | 99% (73) | 100% (7) | 91% (11) |
B.5.4 Commercial sector
Table 16 shows the inspection performance of licensees in the commercial sector. The performance of the sector is shown for the years 2017 to 2021 as a percentage of the inspections that received satisfactory ratings for the SCA. The total number of inspections conducted to assess performance in the SCA appears in parentheses. The number of inspections for the commercial sector is the aggregate for the entire sector.
In light of the small number of inspections in each subsector, a breakdown by subsector is not provided. It would be difficult to identify trends in the subsectors given the low number of licensees in many of them.
| SCA | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Management system | 93% (62) | 97% (41) | 97% (40) | 100% (14) | 92% (26) |
| Operating performance | 94% (67) | 92% (48) | 89% (36) | 94% (18) | 87% (30) |
| Radiation protection | 95% (63) | 100% (46) | 83% (48) | 100% (21) | 90% (29) |
| Security | 94% (53) | 93% (41) | 91% (35) | 90% (10) | 100% (16) |
Appendix C: Enforcement actions issued in 2021
In 2021, CNSC staff issued 10 orders and 1 administrative monetary penalty (AMP) to licensees. Most of the enforcement actions were issued to licensees in the industrial sector, consistent with previous years. The only exception was 1 order that was issued to a non-licensee in unauthorized possession of radiation devices. This order is not included in figure 10 as the non-licensee is not part of any sector. As mentioned in section 4.0 of the report, the increase in enforcement actions in 2021 compared to 2020 was expected given that the number of inspections performed in 2021 also increased.
A complete list of orders issued is included in table 17. Information on the AMP issued is included in table 18. All enforcement actions are closed, and the CNSC is satisfied that the licensees have addressed the conditions of the orders and/or paid the AMPs.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 10: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Medical | 0 | 1 | 0 | 0 | 0 |
| Industrial | 23 | 14 | 9 | 4 | 10 |
| Academic and research | 0 | 0 | 0 | 0 | 0 |
| Commercial | 1 | 1 | 4 | 2 | 0 |
| All sectors combined | 24 | 16 | 13 | 6 | 10 |
| Number of inspections | 944 | 949 | 863 | 371 | 583 |
| Date of issue | Order # | Location | Licensee | Subsector / sector | Order summary | Licensee response | Closed |
|---|---|---|---|---|---|---|---|
| February 15, 2021 | 1223 | 4712 97 Street, Edmonton, Alberta | Focus NDTIS Inc. | Industrial radiography –industrial sector | The order was issued following a CNSC remote inspection of the licensee’s location in Edmonton. The inspection identified several non-compliances with regulatory requirements related to inventory control, exposure device maintenance, records availability and maintenance, worker designation and training, ascertainment of doses and adherence to internal procedures. The inspection identified that the licensee had failed to implement an effective radiation protection program. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | April 22, 2021 |
| March 17, 2021 | 0570 | 9637 45 Avenue NW, Unit 201, Edmonton, Alberta | Canadian Engineering & Inspection Ltd. | Industrial radiography –industrial sector | The order was issued after the CNSC determined that the licensee’s appointed radiation safety officer did not possess the required training and qualifications to effectively manage the licensee’s radiation safety program. | The licensee decided to dismantle its in-house radiography testing program and requested the revocation of its CNSC licence. | April 20, 2021 |
| April 13, 2021 | 1080 | 9720 68 Street SE, Calgary, Alberta | AM Jade Co. | N/A | The company was in unauthorized possession of 4 uncertified Industrial Nucleonic Corp. model PHH-3 nuclear gauges containing greater than 10 exemption quantities of cesium-137 sources. | The devices were transferred to ALARA Consultants for disposal. | September 7, 2021 |
| July 20, 2021 | 1243 | 2051 Williams Parkway, Unit 20, Brampton, Ontario | Davroc Testing Laboratories Inc. | Portable gauge –industrial sector | The order was issued following a CNSC field inspection at a construction site in Innisfil, Ontario, where it was observed that a worker had left a portable nuclear gauge unattended and unsecured without visual oversight. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | August 17, 2021 |
| August 23, 2021 | 1251 | 25 West Beaver Creek Road, Richmond Hill, Ontario | Candec Engineering Consultants Inc. | Portable gauge –industrial sector | The order was issued based on the findings of a CNSC field inspection conducted on July 22, 2021, in Lindsay, Ontario, and a CNSC onsite records inspection conducted on August 23, 2021, at the licensee’s facility in Richmond Hill, Ontario. The field inspection identified non-compliances related to device labelling and transport documentation requirements. The records inspection identified several non-compliances related to worker obligations, worker training, record keeping, leak testing, annual compliance report submissions, radiation detection instrumentation calibrations, posting of signs, emergency procedures, internal procedures and the transportation of the gauges. This included repeat non-compliances from past inspections. In addition, the records inspection identified that the licensee had not met one of its commitments that formed part of the licensing basis. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | September 27, 2021 |
| September 15, 2021 | 0575 | 17 De L'Industrie Street, Saint-Rémi, Quebec | Groupe ABS Inc. | Portable gauge –industrial sector | The order was issued following a CNSC field inspection at a construction site in Montréal, where it was observed that a worker had left a portable nuclear gauge unattended and unsecured. In addition, the inspection identified that the worker was not following internal procedures and was not adequately trained. | The licensee complied with the term of the order to the satisfaction of the CNSC. | October 5, 2021 |
| October 1, 2021 | 1252 | 164 Evans Ave, Toronto, Ontario | Cool Beer Brewing Co. Incorporated | Fixed gauge –industrial sector | The order was issued following a CNSC onsite inspection conducted on September 24, 2021, and a follow-up onsite verification conducted on September 28, 2021, at the licensee’s facility in Toronto. The combined observations from these onsite verification activities identified that there was no trained radiation safety officer (RSO) appointed who could provide an acceptable level of oversight to ensure safe operation and maintenance of the licensee’s fixed nuclear gauge and who could implement the emergency procedures related to this device. The previous RSO had left the company earlier in 2021. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | October 19, 2021 |
| October 4, 2021 | 1338 | 505 Du Parc Technologique Boulevard, Suite 200, Québec City, Quebec | Englobe Corp. | Portable gauge –industrial sector | The order was issued following a CNSC field inspection at a construction site in Montréal, where it was observed that a worker had left a portable nuclear gauge unattended and unsecured. The inspection identified that the worker was not following internal procedures. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | October 26, 2021 |
| October 4, 2021 | 1339 | 97 De La Couronne Street, Repentigny, Quebec | 9395-8049 Québec inc., operating through it subsidiary Solmatech inc. | Portable gauge –industrial sector | The order was issued following a CNSC field inspection at a construction site in Montréal, where it was observed that a worker had left a portable nuclear gauge unattended and unsecured. The inspection identified that the worker was not following internal procedures. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | November 8, 2021 |
| October 14, 2021 | 1340 | 433 Chabanel Street West, Montréal, Quebec | FNX-INNOV Inc. | Portable gauge –industrial sector | The order was issued following a CNSC field inspection at a construction site in Laval, Quebec, where it was observed that a worker had left a portable nuclear gauge unattended and unsecured. The inspection identified that the worker was not following internal procedures. | The licensee complied with the terms of the order to the satisfaction of the CNSC. | May 10, 2022 |
| Date of issue | AMP | Licensee | Subsector / sector | Amount | AMP description | Closed |
|---|---|---|---|---|---|---|
| March 1, 2021 | 2021-AMP-01 | Catalyst Paper Corporation | Fixed gauge –industrial sector | $2,000 | Failure to comply with provision 48(c) of the Nuclear Safety and Control Act: Failure to comply with a condition of the licence. Specifically, failure to comply with licence condition 2052-3 related to entry into a vessel or hopper fitted with a fixed gauge. | Paid on March 26, 2021 |
Appendix D: Doses to workers
Occupational doses were reported by licensees for a total of 56,040 workers in the 4 sectors in 2021. Of those workers, 24,066 were nuclear energy workers (NEWs). The difference in doses to workers among sectors reflects the nature of the various activities within those sectors. Figure 11 shows the doses received by non-NEWs monitored in 2021, with 93% reported as having received doses less than or equal to 0.5 mSv. Figure 12 shows the doses received by NEWs monitored in 2021. Based on the reported doses for NEWs, only 14% received a dose greater than 1 mSv, and only about 1% received a dose above 5 mSv. Figure 13 shows the doses to NEWs from 2017 to 2021.
As the figures demonstrate, doses overall have remained low and stable over the years. This is an indication that industry has successfully kept doses as low as reasonably achievable. Given the nature of the work performed in many cases, it is inevitable that some workers will receive a dose. The constancy year over year indicates that doses have likely achieved a state of equilibrium – changes in operational procedures will likely not yield any significant improvement in dose.
More information on unusual doses is provided in section 5 of this report.
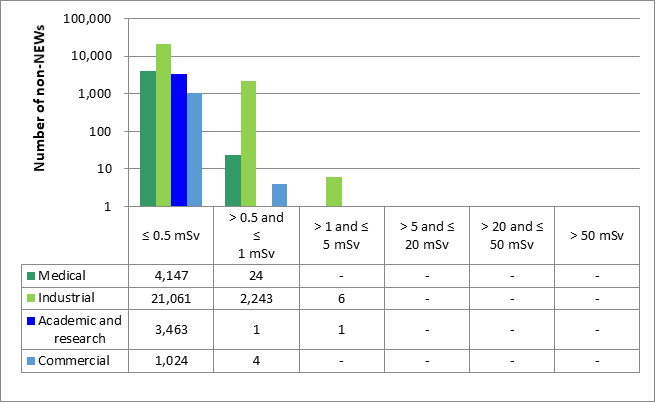
Figure 11: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Medical | 4,147 | 24 | 0 | 0 | 0 | 0 |
| Industrial | 21,061 | 2,243 | 6 | 0 | 0 | 0 |
| Academic and research | 3,463 | 1 | 1 | 0 | 0 | 0 |
| Commercial | 1,024 | 4 | 0 | 0 | 0 | 0 |
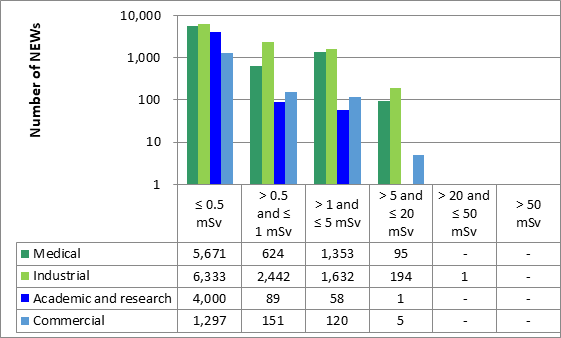
Figure 12: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Medical | 5,671 | 624 | 1,353 | 95 | 0 | 0 |
| Industrial | 6,333 | 2,442 | 1,632 | 194 | 1 | 0 |
| Academic and research | 4,000 | 89 | 58 | 1 | 0 | 0 |
| Commercial | 1,297 | 151 | 120 | 5 | 0 | 0 |
One NEW, working in the portable gauge subsector, was reported to have received a dose of 22.3 mSv, which was unexpected based on the work performed. The worker was observed while using the gauge and was deemed to be operating it safely. Upon further investigation, the licensee determined that the worker had been storing his dosimeter on top of the portable gauge case when not in use, which accounted for the unusual dosimeter readings. Even though the dose received was below the regulatory limits for a NEW, the worker was removed from work that required the use of a nuclear gauge as a personal safety precaution during the investigation. The worker has now moved into a different position, where he will no longer be using a portable gauge. No dose change request was submitted.
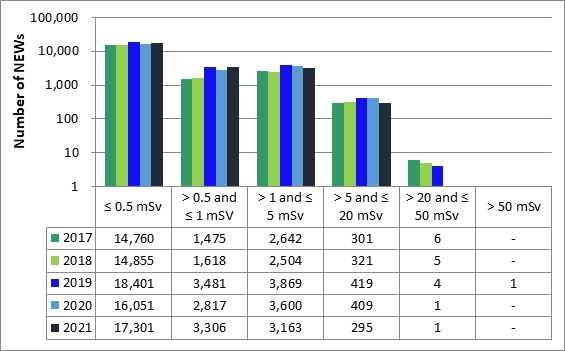
Figure 13: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| 2017 | 14,760 | 1,475 | 2,642 | 301 | 6 | 0 |
| 2018 | 14,855 | 1,618 | 2,504 | 321 | 5 | 0 |
| 2019 | 18,401 | 3,481 | 3,869 | 419 | 4 | 1 |
| 2020 | 16,051 | 2,817 | 3,600 | 409 | 1 | 0 |
| 2021 | 17,301 | 3,306 | 3,163 | 295 | 1 | 0 |
D.1 Medical sector
Figure 14 shows the doses received by NEWs in the medical sector, as reported to the CNSC for 2021. Note that the total number of NEWs shown in the “Medical sector” row is the aggregate for the entire sector, including subsectors not highlighted. Results are similar to past years.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 14: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Nuclear medicine | 2,457 | 597 | 1,327 | 90 | 0 | 0 |
| Radiation therapy | 3,067 | 18 | 14 | 2 | 0 | 0 |
| Veterinary nuclear medicine | 110 | 5 | 4 | 2 | 0 | 0 |
| Entire medical sector | 5,671 | 624 | 1,353 | 95 | 0 | 0 |
D.2 Industrial sector
Figure 15 shows the doses received by NEWs in the industrial sector, as reported to the CNSC for 2021. Note that the total number of NEWs shown in the “Industrial sector” row is the aggregate for the entire sector, including subsectors not highlighted. Results are similar to past years.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 15: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Portable gauge | 3,048 | 2,077 | 919 | 32 | 1 | 0 |
| Fixed gauge | 875 | 11 | 0 | 0 | 0 | 0 |
| Industrial radiography | 1,406 | 305 | 666 | 157 | 0 | 0 |
| Oil-well logging | 933 | 45 | 45 | 5 | 0 | 0 |
| Entire industrial sector | 6,321 | 2,442 | 1,632 | 194 | 1 | 0 |
D.3 Academic and research sector
Figure 16 shows the doses received by NEWs in the academic and research sector, as reported to the CNSC for 2021. Note that the total number of NEWs shown in the “Academic and research sector” row is the aggregate for the entire sector, including subsectors not highlighted. Results are similar to past years.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 16: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Laboratory studies and the consolidated use of nuclear substances | 3,436 | 33 | 26 | 0 | 0 | 0 |
| Entire academic and research sector | 4,000 | 89 | 58 | 1 | 0 | 0 |
D.4 Commercial sector
Figure 17 shows the doses received by NEWs in the commercial sector, as reported to the CNSC for 2021. Note that the total number of NEWs shown in the “Commercial sector” row is the aggregate for the entire sector, including subsectors not highlighted. Results are similar to past years.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 17: Text version
| N/A | Less than or equal to 0.5 mSv | Greater than 0.5 and less than or equal to 1 mSv | Greater than 1 and less than or equal to 5 mSv | Greater than 5 and less than or equal to 20 mSv | Greater than 20 and less than or equal to 50 mSv | Greater than 50 mSv |
|---|---|---|---|---|---|---|
| Isotope production | 167 | 15 | 6 | 0 | 0 | 0 |
| Processing of nuclear substances | 239 | 42 | 50 | 2 | 0 | 0 |
| Distribution | 42 | 1 | 4 | 2 | 0 | 0 |
| Servicing | 274 | 26 | 31 | 1 | 0 | 0 |
| Calibration | 74 | 1 | 0 | 0 | 0 | 0 |
| Waste nuclear substance | 446 | 66 | 27 | 0 | 0 | 0 |
| Entire commercial sector | 1,297 | 151 | 120 | 5 | 0 | 0 |
Appendix E: Reportable events
In 2021, CNSC staff received from licensees 197 notifications of events related to nuclear substances and prescribed equipment. Staff assessed 171 of these to be reportable events. Notifications not considered reportable events include action level exceedances, fishing operations (well-logging), and flooding where no nuclear substances or prescribed equipment have been affected. Of the 171 reportable events, 165 were rated as level 0 (no safety significance) on the International Nuclear and Radiological Event Scale (INES) and 6 were rated as INES level 1 (anomaly). For all events reported, licensees implemented appropriate response measures to mitigate the impacts and to limit radiation exposure to workers and the public. CNSC staff reviewed the response measures and found them to be satisfactory.
.png/object?subscription-key=3ff0910c6c54489abc34bc5b7d773be0)
Figure 18: Text version
| N/A | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Malfunctioning or damaged devices | 50 | 66 | 67 | 47 | 51 |
| Spills, contamination or release | 19 | 20 | 19 | 16 | 26 |
| Lost, stolen or found nuclear substances | 18 | 19 | 6 | 8 | 10 |
| Packaging and transport | 33 | 56 | 46 | 31 | 41 |
| Breach of security | 11 | 16 | 21 | 14 | 31 |
| Unplanned exposure | 13 | 13 | 22 | 15 | 6 |
| Other | 2 | 5 | 3 | 4 | 6 |
| All reported events | 146 | 195 | 183 | 135 | 171 |
Unplanned exposures may include individuals crossing safety barriers while industrial radiography is occurring, skin contamination events, and any events where regulatory limits are exceeded.
As noted in section 6.0, event data related to transport is a more meaningful indicator than licensee performance ratings for the packaging and transport SCA. Out of the 171 reportable events in 2021, 41 (24%) were related to transport. For the most part (76%), transport events were related to minor motor vehicle accidents (MVAs) where there was no damage to the package being transported and no injury to the driver. None of the transport-related events were considered risk-significant; all were rated as INES level 0. Given the large number of packages containing radioactive material that are shipped on a regular basis in Canada, the small number of transport events reported in 2021 – all of which were of low risk-significance – provides an indicator of the overall level of safety of this activity.
Table 19: Reportable events by sector and subsector in 2021
| Subsector | Malfunctioning or damaged devices | Spills, contamination or release | Lost, stolen or found nuclear substances | Packaging and transport | Breach of security | Unplanned exposure | Other |
|---|---|---|---|---|---|---|---|
| Isotope production | 3 | 6 | 0 | 1 | 0 | 0 | 0 |
| Processing of nuclear substances | 0 | 1 | 0 | 12 | 0 | 1 | 0 |
| Distribution | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Servicing | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Calibration | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Waste nuclear substance | 0 | 3 | 0 | 2 | 5 | 0 | 6 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsector | Malfunctioning or damaged devices | Spills, contamination or release | Lost, stolen or found nuclear substances | Packaging and transport | Breach of security | Unplanned exposure | Other |
|---|---|---|---|---|---|---|---|
| Nuclear medicine | 1 | 15 | 2 | 5 | 3 | 1 | 0 |
| Radiation therapy | 2 | 0 | 1 | 0 | 10 | 0 | 0 |
| Veterinary nuclear medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsector | Malfunctioning or damaged devices | Spills, contamination or release | Lost, stolen or found nuclear substances | Packaging and transport | Breach of security | Unplanned exposure | Other |
|---|---|---|---|---|---|---|---|
| Portable gauge | 17 | 0 | 5 | 16 | 6 | 0 | 0 |
| Fixed gauge | 22 | 0 | 2 | 0 | 1 | 0 | 0 |
| Industrial radiography | 4 | 0 | 0 | 3 | 0 | 4 | 0 |
| Oil-well logging | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsector | Malfunctioning or damaged devices | Spills, contamination or release | Lost, stolen or found nuclear substances | Packaging and transport | Breach of security | Unplanned exposure | Other |
|---|---|---|---|---|---|---|---|
| Laboratory studies and consolidated use | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Subsector | Malfunctioning or damaged devices | Spills, contamination or release | Lost, stolen or found nuclear substances | Packaging and transport | Breach of security | Unplanned exposure | Other |
|---|---|---|---|---|---|---|---|
| Total events, all sectors combined | 51 | 26 | 10 | 41 | 31 | 6 | 6 |
Note: Where a specific subsector is not highlighted, the number of events is captured under “other” in each sector.
| Event ID | Date reported | INES rating | Event type | Sector | Event summary |
|---|---|---|---|---|---|
| 5111 | January 11 | 0 | Transport | Industrial | A portable gauge fell off a forklift during unloading. The gauge was sent for inspection and maintenance prior to use. |
| 5115 | January 14 | 0 | Damaged device | Industrial | An exposure device was run over by a truck. Damage was limited to the camera crank handle and the cable. There was no loss of containment and no overexposures as a result of this event. |
| WNSL-1 | January 15 | 0 | Transport | Commercial | A shipment meant to contain only empty low-level radioactive waste (LLRW) bins also contained 1 bin of actual LLRW. The full bin was properly packaged and returned. There were no overexposures as a result of this event. |
| 5122 | January 18 | 0 | Breach of security | Medical | The door of a hot lab was left unlocked for a period of 24 hours after a delivery. All nuclear substances were accounted for. |
| 5124 | January 21 | 0 | Transport – MVA | Commercial | A vehicle transporting empty excepted packages was involved in an MVAThere was no damage to the packages. |
| 5126 | January 26 | 0 | Breach of security | Medical | Nuclear substances were left unattended in a hallway for a brief period. All nuclear substances were accounted for. |
| 5129 | January 27 | 0 | Damaged device | Industrial | A fixed gauge was not functioning properly due to debris collecting in the source housing. The sealed source was removed and reinstalled by a licensed third party into a new source holder and then put back into operation. |
| 5131 | February 1 | 0 | Damaged device | Industrial | A fixed gauge shutter was stuck in the open position. The shutter was successfully repaired. There were no overexposures as a result of this event. |
| 5134 | February 2 | 0 | Damaged device | Industrial | An exposure device was dropped from scaffolding, causing minor damage to the casing. The exposure device was sent for repair and leak testing. There were no overexposures as a result of this event. |
| 5136 | February 4 | 0 | Transport | Commercial | Two Type A packages were damaged by a forklift. There was no loss of containment and no external contamination on the packages. Both packages were able to be delivered. |
| 5149 | February 5 | 0 | Transport – MVA | Industrial | A vehicle transporting an exposure device was involved in an MVA. There was no damage to the exposure device. |
| 5140 | February 11 | 0 | Release | Commercial | There was an unplanned release of fluorine-18 to the environment. There were no overexposures to workers or the public as a result of this event. |
| 5143 | February 16 | 0 | Transport – MVA | Commercial | A vehicle transporting medical isotopes was involved in an MVA. There was no damage to the packages. |
| 5144 | February 16 | 0 | Spill | Medical | There was a spill greater than 100 EQ of technetium-99m. There was no skin contamination and no overexposures. |
| 5146 | February 17 | 0 | Damaged device | Industrial | A portable gauge was run over by a truck on a construction site. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of the event. |
| WNSL-2 | February 25 | 0 | Security | Commercial | Security-related event – Confidential |
| 5154 | February 27 | 0 | Damaged device | Industrial | A portable gauge was run over by an asphalt roller on a construction site. The damage was limited to the handle of the gauge. There was no loss of containment and no overexposures as a result of the event. |
| 5157 | March 3 | 0 | Damaged device | Medical | The handle on a rubidium-82 generator was stuck, preventing use of the generator. The generator was removed from service and stored for decay prior to being returned to the supplier. There was no contamination and no releases as a result of this event. |
| 5158 | March 4 | 0 | Transport | Medical | A seal on a Type A package containing technetium-99m was broken upon receipt of the package. All nuclear substances were accounted for but were not used by the licensee. |
| 5159 | March 4 | 0 | Damaged device | Industrial | An exposure device fell from height, causing damage to the source tube. The exposure device was sent for repair and inspection. There were no overexposures as a result of the event. |
| 5160 | March 4 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5161 | March 5 | 0 | Device malfunction | Industrial | A malfunctioning fixed gauge was discovered as part of a routine inspection. The fixed gauge was serviced onsite and is now functional. There was no loss of containment and no overexposures as a result of the event. |
| 5166 | March 5 | 0 | Transport | Commercial | A Type A package was damaged during transport. There was no loss of containment and no external contamination on the package. |
| 5164 | March 6 | 0 | Device malfunction | Industrial | An exposure device malfunctioned during use. It was deemed not repairable and was disposed of appropriately. There were no overexposures as a result of the event. |
| 5165 | March 8 | 0 | Transport – MVA | Commercial | A vehicle transporting empty excepted packages was involved in a minor MVA. There was no damage to the packages. |
| WNSL-3 | March 10 | 0 | Transport – MVA | Commercial | A truck rolled backwards down a small grade, broke through a storage fence, and connected with a transport container. There was no damage to the container or the contents. |
| WNSL-4 | March 10 | 0 | Equipment malfunction | Commercial | Air samplers were sampling local air instead of air released from the stacks as a result of an equipment malfunction. Based on the work performed during that time, there were no releases that would have exceeded action levels. The air samplers were replaced. |
| 5167 | March 15 | 0 | Contamination | Medical | A NEW received skin contamination with technetium-99m. The extremity dose to the right hand was 88.5 mSv, which is below regulatory limits. |
| 5169 | March 18 | 0 | Damaged device | Industrial | A fixed gauge was displaced by about 2 inches from its expected position. The only noticeable damage was to the housing paint. There was no loss of containment and no overexposures as a result of this event. |
| 5170 | March 19 | 0 | Transport | Medical | Upon receipt of a Type A package containing zirconium-89, contamination on the external surface was discovered. The vial within the package was empty of its contents. There was no contamination found on any surface where the package transited or on any person who handled the package. The package was stored for decay prior to disposal. |
| 5177 | March 22 | 0 | Damaged device | Industrial | A fixed gauge malfunctioned because of a damaged tube between the source window and the sensor. There were no overexposures as a result of this event. |
| 5175 | March 23 | 0 | Transport – MVA | Commercial | A vehicle transporting spent technetium-99m generators was involved in a minor MVA. There was no damage to the packages. |
| 5180 | April 5 | 1 | Stolen device | Industrial | A portable gauge (Category 4) in its Type A package was stolen from a secured industrial site along with other tools. The portable gauge has not been recovered. |
| 5185 | April 5 | 0 | Device malfunction | Commercial | A status light for a hot cell associated with cyclotron operations malfunctioned. Repairs were identified and completed. |
| 5194 | April 6 | 0 | Found device | Industrial | A member of the public was in unauthorized possession of 4 historical fixed gauges that were discovered in an equipment inventory check. These were likely the remains of a previous occupant at the site. The sources were shielded and inaccessible to the public. An order was issued by the CNSC to dispose of the gauges through an authorized CNSC licensee. There were no overexposures related to this event. |
| 5184 | April 7 | 0 | Damaged device | Industrial | A portable gauge was run over by a truck on a construction site. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of the event. |
| 5187 | April 9 | 0 | Breach of security | Academic and research | A security system in an administrative area was left unarmed. There was no actual attempted intrusion. All other systems were in place. |
| 5195 | April 20 | 0 | Breach of security | Academic and research | A security system was not armed over a weekend, likely because of a power disruption. There was no actual attempted intrusion. Other security systems remained in place. |
| 5196 | April 20 | 0 | Unplanned exposure | Commercial | A spill greater than 100 EQ of iodine-131 occurred in a radiation-shielded box, under a vented hood. The subsequent clean-up resulted in an unplanned exposure to a NEW of 29 mSv (effective dose) and a committed equivalent dose to the thyroid of 560 mSv. Both of these are below regulatory limits. A verbal EIR was presented to the Commission in April 2021, with subsequent follow-ups in October 2021 and March 2022. |
| 5204 | April 20 | 0 | Device malfunction | Industrial | Elevated dose rates were discovered around a fixed gauge. Physical barriers have been installed to ensure that there are no resulting overexposures, and the gauge will be replaced during the next shutdown. |
| WNSL-5 | April 25 | 0 | Security | Commercial | Security-related event – Confidential |
| 5214 | April 27 | 0 | Device malfunction | Medical | A medical linear accelerator turned on unexpectedly during safety checks because of a faulty board. No one was in the room when it came on. There were no overexposures related to this event. |
| 5206 | April 29 | 0 | Breach of security | Medical | Because of an electronic network upgrade, no live signal from one particular surveillance device was available to security personnel. The licensee confirmed, however, that all the essential barriers remained in place. The licensee implemented corrective actions and repairs have been done. |
| 5200 | April 30 | 0 | Unplanned exposure | Industrial | Four non-NEWs were unknowingly in a vessel, inside a barrier set for industrial radiography exposures, at the time when 2 exposures were taken. A re-enactment of the event confirmed that there were no overexposures as a result of this event. |
| 5201 | April 30 | 0 | Damaged device | Industrial | A fixed gauge was damaged when the mounting support broke and the gauge fell from about 2.5 metres. The shutter was closed and locked and the gauge placed in secure storage. There was no loss of containment and no overexposures related to this event. |
| 5203 | May 3 | 0 | Spill | Commercial | A spill greater than 100 EQ of strontium-82 occurred in a clean room. The room was decontaminated and there were no overexposures as a result of this event. |
| WNSL-6 | May 6 | 0 | Contamination | Commercial | The equipment meant to separate contaminated from non-contaminated coveralls designated for repair malfunctioned. Some contaminated coveralls were sent for repair to a non-licenced facility. Given the low levels of contamination (just above the release limits), no unplanned exposures were anticipated. |
| 5209 | May 9 | 1 | Stolen device | Industrial | A portable gauge (Category 4 sealed source) in its Type A package was stolen from a parked vehicle. The portable gauge has not been recovered. |
| 5211 | May 10 | 0 | Breach of security | Industrial | A lock on a security cage around a fixed gauge appeared to have been tampered with. Further investigation revealed that the lock likely wore through the hasp as a result of constant vibration. There was no unauthorized entry to the building on the day in question, and all nuclear substances are accounted for. |
| 5216 | May 18 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5217 | May 19 | 0 | Unplanned exposure | Medical | The licensee reported that a NEW had received a dose of 146 mSv. Upon investigation, it was deemed to be a non-personal dose, and a dose change request was submitted and approved. An EIR was reported to the Commission in June 2021, with a follow-up in October. |
| 5218 | May 19 | 0 | Spill | Medical | A spill greater than 100 EQ of fluorine-18 occurred while injecting a patient. There was no skin contamination and no overexposures. |
| 5219 | May 20 | 0 | Breach of security | Industrial | A portable gauge was left unattended on a restricted-access work site. The source was shielded, and the trigger locked. The worker realized their error and contacted someone onsite to secure the gauge. There were no overexposures related to this event. |
| 5306 | May 24 | 0 | Damaged device | Industrial | A neutron generator was damaged as it was being recovered from a well. The device was sent for disposal. There were no unplanned exposures as a result of this event. |
| 5221 | May 25 | 0 | Breach of security | Academic and research | A security system was not armed over a weekend, likely because of a faulty electrical panel. There was no actual attempted intrusion. Other security systems remained in place. |
| WNSL-7 | May 26 | 0 | Equipment malfunction | Commercial | A hand and foot radiation monitor was found to be out of calibration for 5 days. No adjustments were required once calibrated. No contamination was missed during this period. |
| WNSL-8 | May 27 | 0 | Equipment malfunction | Commercial | A sprinkler system was out of service because of a municipal waterline failure and shutdown. |
| 5226 | May 27 | 0 | Breach of security | Industrial | A portable gauge was left unsecured in the back of a truck. Another worker noticed and installed a lock and notified the radiation safety officer. |
| 5230 | June 1 | 0 | Breach of security | Industrial | A portable gauge was left unattended on a restricted-access work site. The source was shielded, and the trigger locked. The site supervisor stayed close to the gauge until the licensee returned. There were no overexposures related to this event. |
| 5233 | June 3 | 0 | Spill | Medical | A spill greater than 100 EQ of fluorine-18 occurred and included contamination of a NEW. After decontamination, the estimated maximum dose received by the NEW was 406 mSv. There were no overexposures related to this event. |
| 5234 | June 3 | 0 | Damaged device | Industrial | A portable gauge was run over by a van on a construction site. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of this event. |
| 5245 | June 3 | 0 | Contamination | Commercial | A vial of fluorine-18 broke in its shielded container, leading to contamination of the surrounding area. There was no skin contamination. There were no overexposures related to this event. |
| 5238 | June 7 | 0 | Unplanned exposure | Industrial | A worker (member of the public) at a job site crossed a barrier during an industrial radiography exposure. Based on a re-enactment, there was no overexposure to the member of the public as a result of this event. |
| 5243 | June 9 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was stuck in the open position. Given the positioning of the gauge, there was no risk to the workers. The gauge was repaired, and the shutter is now functional. |
| 5246 | June 11 | 0 | Unplanned exposure | Industrial | A worker (member of the public) at a job site crossed a barrier during an industrial radiography exposure. Based on a re-enactment, there was no overexposure to the member of the public as a result of this event. |
| 5247 | June 11 | 0 | Damaged device | Industrial | A portable gauge was run over by a bulldozer on a construction site. The gauge was removed from service and sent for disposal. There was no loss of containment and no overexposures as a result of this event. |
| 5248 | June 11 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| WNSL-9 | June 14 | 0 | Death at a licensee facility | Commercial | A sub-contactor was fatally injured while providing a service to the licensee. The fatality was industrial in nature. The Ministry of Labour, Training and Skills Development for the Province of Ontario was notified, and it began an investigation on June 14, 2021. An EIR was presented to the Commission during the October 5, 2021, meeting. |
| 5250 | June 16 | 0 | Spill | Medical | A spill greater than 100 EQ of technetium-99m occurred when a vial was dropped on the floor. There was no personal contamination and no overexposures as a result of this event. |
| 5251 | June 17 | 0 | Transport – MVA | Commercial | A vehicle transporting empty packages with trace amounts of technetium-99m was involved in a minor MVA. There was no damage to the packages. |
| 5252 | June 17 | 0 | Contamination | Commercial | Contamination of a worker’s shoe was discovered during an exit scan. There was no contamination outside the facility. The area was decontaminated. |
| 5253 | June 17 | 0 | Contamination | Industrial | A NEW was contaminated with bromine-82 while performing work on a pipeline. The NEW received a maximum equivalent (skin) dose of 20 mSv. There were no overexposures as a result of this event. |
| 5257 | June 19 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5265 | June 23 | 0 | Damaged device | Industrial | A portable gauge was run over by an asphalt roller on a construction site, damaging the cover and the handle. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of this event. |
| 5267 | June 23 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by a piece of equipment on a construction site. The gauge was removed from service and sent for disposal. There was no loss of containment and no overexposures as a result of this event. |
| 5269 | June 24 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was stuck in the open position. The gauge was dismounted and sent for disposal. There were no overexposures as a result of this event. |
| 5272 | June 29 | 0 | Transport | Medical | A package containing an Mo-99/Tc-99m generator was received with minor damage to the package. The generator was intact and there was no loss of containment as a result of this event. |
| 5273 | June 30 | 0 | Lost material | Medical | There was a lost iodine-125 seed (Category 5 sealed source). The source was not recovered. |
| 5274 | July 3 | 0 | Transport – MVA | Commercial | A truck carrying pallets of smoke detectors was involved in an MVA. The outer case packs were damaged but the smoke detectors were intact. |
| 5384 | July 5 | 0 | Contamination | Commercial | A spill greater than 100 EQ of fluorine-18 occurred. Three NEWs were contaminated; however, there were no overexposures as a result of this event. |
| 5275 | July 7 | 0 | Transport | Medical | An excepted package with minor damage to the package was received. The source was intact, and there was no loss of containment as a result of this event. |
| 5276 | July 7 | 0 | Transport – MVA | Commercial | A vehicle transporting technetium-99m in a Type A package as well as empty excepted packages was involved in a minor MVA. There was no damage to the packages. |
| 5278 | July 9 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by a vehicle on a construction site. There was no loss of containment and no overexposures as a result of this event. |
| 5280 | July 14 | 1 | Stolen device | Industrial | A vehicle with a portable gauge (Category 4 sealed source) secured in its Type A package was stolen from a worker’s residence. The portable gauge has not been recovered. |
| 5281 | July 14 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a truck on a construction site. The gauge was removed from service and sent for disposal. There was no loss of containment and no overexposures as a result of this event. |
| 5282 | July 14 | 0 | Spill | Commercial | There was a minor leak of carbon-11. There was no skin contamination and no overexposures as a result of this event. |
| 5284 | July 15 | 0 | Device malfunction | Industrial | The shutter on a portable gauge was stuck in the open position. The gauge was cleaned and serviced prior to being returned to service. There were no overexposures as a result of this event. |
| 5283 | July 19 | 0 | Spill | Medical | There were 2 spills greater than 100 EQ of technetium-99m each. There was no skin contamination and no overexposures as a result of these events. |
| 5286 | July 19 | 0 | Device malfunction | Medical | A brachytherapy unit malfunctioned during patient treatment. The unit was returned to service once reset. There were no overexposures as a result of this event. |
| 5289 | July 20 | 0 | Contamination | Medical | A NEW inadvertently poked themselves with a needle contaminated with technetium-99m. There was no overexposure as a result of this event. |
| 5288 | July 22 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| WNSL-10 | July 26 | 0 | Bankruptcy | Commercial | A licensee declared bankruptcy. All nuclear substances have been secured. An EIR was presented to the Commission during the October 5, 2021, meeting, and an update was provided during the November 23, 2021, meeting as part of the Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2020. |
| 5295 | July 30 | 0 | Spill | Medical | A spill greater than 100 EQ of fluorine-18 occurred. There was no skin contamination and no overexposures as a result of this event. |
| 5303 | August 2 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5320 | August 2 | 0 | Breach of security | Industrial | A portable gauge was left unattended and unsecured in a pickup truck parked for the night. The gauge was still in its case when the breach was discovered. |
| 5302 | August 5 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5309 | August 6 | 0 | Damaged device | Industrial | The shutter of a fixed gauge was stuck in the open position. Given the position of the gauge, and with supplemental shielding in place, the licensee continued to use the gauge until it was replaced. The damaged gauge was transferred to a service company for eventual disposal. There were no unplanned exposures as a result of this event. |
| 5300 | August 9 | 0 | Device malfunction | Industrial | The shutter on a fixed gauge was stuck in the open position. The gauge was dismounted and sent for disposal. There were no overexposures as a result of this event. |
| 5305 | August 10 | 0 | Device malfunction | Commercial | A cyclotron shut down unexpectedly during a power outage when it should have been connected to a back-up power supply. The equipment has been returned to service. |
| WNSL-11 | August 14 | 0 | Equipment malfunction | Commercial | A fire alarm was falsely triggered. It was confirmed that there was no fire in the area. |
| 5241 | August 17 | 0 | Spill | Commercial | A spill greater than 100 EQ of fluorine-18 occurred during a transfer procedure. There was no skin contamination of the NEW. There were no overexposures related to this event. |
| 5308 | August 19 | 0 | Damaged device | Industrial | The weld between a mounting plate and the source holder of a fixed gauge was damaged. The fixed gauge was sent for disposal. There were no unplanned exposures as a result of this event. |
| 5311 | August 23 | 0 | Damaged device | Medical | A spring was broken on one of the sources in a self-shielded irradiator. The irradiator was repaired and returned to service. |
| 5318 | September 1 | 0 | Breach of security | Industrial | Two portable gauges were left unattended by a delivery company at an incorrect address. Once tracked by the licensee, the gauges were recovered and placed in secure storage. The gauges were intact, and there were no unplanned exposures as a result of this event. |
| 5319 | September 1 | 0 | Spill | Medical | A spill greater than 100 EQ of fluorine-18 occurred. There was no skin contamination and no overexposures as a result of this event. |
| 5321 | September 2 | 0 | Device malfunction | Industrial | The stop bolt on a portable gauge handle was loose, causing the handle to fall off the gauge. The issue was addressed. There were no overexposures as a result of this event. |
| 5322 | September 3 | 0 | Contamination | Medical | Three check sources were found to be contaminated with cesium-137 during routine wipes upon receipt of the sources. The package was not contaminated. There was no skin contamination and no overexposures as a result of this event. |
| 5324 | September 4 | 0 | Breach of security | Academic and research | Concrete blocks were inadvertently removed from a hallway wall during renovations. This wall was part of the outer room, which houses an inner room where prescribed equipment is located. The wall has since been permanently repaired. There was no loss of nuclear substances and no unplanned exposures as a result of this event. |
| 5326 | September 7 | 0 | Transport – MVA | Commercial | A vehicle transporting empty cases with trace amounts of technetium-99m was involved in a minor MVA. There was no damage to the packages. |
| 5331 | September 13 | 0 | Transport – MVA | Industrial | A vehicle transporting an industrial radiography camera was involved in an MVA. There was no damage to the industrial radiography camera. |
| 5332 | September 14 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in a minor MVA. There was no damage to the portable gauge. |
| 5334 | September 15 | 0 | Damaged device | Industrial | A padlock ring used to lock the handle that controls the shutter on a fixed gauge was damaged. The gauge was removed from service. There were no overexposures as a result of this event. |
| 5336 | September 17 | 0 | Damaged device | Industrial | Two fixed gauges with malfunctioning shutters were discovered. Both gauges were removed from service. There were no overexposures as a result of this event. |
| 5337 | September 21 | 0 | Breach of security | Medical | An alarm was not set for a brief period on a room that contains sealed sources. The nuclear substances were undisturbed, and the alarm was reactivated. There was no actual attempted intrusion. |
| 5338 | September 21 | 0 | Transport – MVA | Commercial | A vehicle transporting a small amount of technetium-99m in multiple packages was involved in a minor MVA. There was no damage to the packages. |
| 5341 | September 22 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in a minor MVA. There was no damage to the portable gauge. |
| 5340 | September 23 | 0 | Transport – MVA | Industrial | A vehicle transporting an industrial radiography camera was involved in an MVA. There was no damage to the camera. |
| 5343 | September 27 | 0 | Lost device | Medical | A brachytherapy plaque containing iodine-125 seeds was lost. The plaque was not recovered. No overexposures are anticipated as a result of this event. |
| 5344 | September 28 | 0 | Damaged device | Industrial | A portable gauge was run over by an excavator on a construction site, damaging its plastic cover. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of the event |
| 5345 | September 28 | 0 | Breach of security | Academic and research | A motion sensor was disarmed for a period of 45 minutes. The system was reset and confirmed to be functional. All nuclear substances were accounted for. |
| 5346 | September 29 | 0 | Breach of security | Industrial | A portable gauge was left unattended on a secured work site. The site supervisor stayed with the gauge until the licensee returned. The gauge showed no signs of damage or tampering, and there were no unplanned exposures as a result of this event. |
| 5348 | September 30 | 0 | Breach of security | Medical | A brachytherapy source was left unattended and unsecured overnight. The source was intact, in its package, when discovered. It was then secured properly. |
| 5349 | September 30 | 0 | Transport – MVA | Commercial | A vehicle transporting technetium-99m in multiple packages was involved in an MVA. There was no damage to the packages. |
| WNSL-12 | October 1 | 0 | Release | Commercial | A holding tank of water from laundry was inadvertently discharged to the sewer. Sampling prior to the discharge indicated that any release of nuclear substances was below derived release limits and action levels. |
| 5354 | October 6 | 0 | Damaged device | Industrial | The rod to operate the shutter on a fixed gauge was difficult to use. The gauge has been repaired. There were no overexposures as a result of this event. |
| 5355 | October 7 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5356 | October 7 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| WNSL-13 | October 8 | 0 | Breach of security / Sabotage | Commercial | A disgruntled employee attempted to sabotage some of the radiation protection program components. There were no environmental releases, no overexposures and no health and safety issues raised as a result of this event. |
| 5357 | October 13 | 1 | Lost device | Industrial | A fixed gauge (Category 4 sealed source) was reported lost. The gauge has not been recovered. |
| 5358 | October 13 | 0 | Device malfunction | Commercial | A spill greater than 100 EQ of fluorine-18 occurred. There was no skin contamination and no overexposures as a result of this event. |
| 5371 | October 14 | 0 | Breach of security | Medical | As part of regular checks of the security systems, it was noted that the alarm system was not functional. The system was repaired while awaiting the installation of a new system. There was no actual attempted intrusion. |
| 5359 | October 15 | 0 | Device malfunction | Industrial | Two fixed gauges with shutters stuck in the open position were reported. The gauges were repaired. There were no overexposures as a result of this event. |
| WNSL-14 | October 18 | 0 | Security | Commercial | Security-related event – Confidential |
| 5360 | October 19 | 1 | Stolen device | Industrial | A secured portable gauge was stolen from a parked vehicle. The gauge was subsequently found and returned to the licensee. |
| 5361 | October 19 | 0 | Transport | Medical | A licensee received a damaged package containing medical isotopes. The contents of the package were intact. There was no contamination as a result of this event. |
| 5363 | October 20 | 0 | Breach of security | Medical | Untrained cleaning staff had access to a hot lab. Access has been removed. |
| 5365 | October 21 | 1 | Stolen device | Industrial | A vehicle with a portable gauge inside was stolen during the night from a worker’s residence. The gauge was recovered and showed no signs of tampering. |
| 5366 | October 21 | 0 | Spill | Medical | A spill greater than 100 EQ of technetium-99m occurred. The technologist was contaminated; however, there were no overexposures as a result of this event. |
| 5369 | October 21 | 0 | Damaged device | Industrial | Welds holding 2 fixed gauges to the base plate were cracked. Both gauges were removed from service and sent for disposal. There were no unplanned exposures as a result of this event. |
| 5373 | October 26 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5387 | November 5 | 0 | Breach of security | Medical | A treatment room containing prescribed equipment was left unsecured with the motion sensor disarmed. There was no actual attempted intrusion. |
| 5409 | November 5 | 0 | Device malfunction | Industrial | The shutter of a portable gauge was stuck in a partially open position. The gauge was removed from service and sent for repair. There were no overexposures as a result of this event. |
| 5390 | November 9 | 0 | Lost material | Medical | A cobalt-57 sealed source was reported missing. The source was recovered and sent for disposal. |
| 5391 | November 9 | 0 | Spill | Medical | An iodine-125 seed was accidently cut in half. The pieces were collected and stored securely. There was no skin contamination as a result of this event. |
| 5392 | November 10 | 0 | Damaged device | Industrial | A portable gauge was damaged by a reversing truck. Only the casing of the gauge was damaged. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of the event. |
| 5395 | November 10 | 0 | Transport – MVA | Commercial | A vehicle transporting excepted packages with trace amounts of technetium-99m was involved in a minor MVA. There was no damage to the packages. |
| 5396 | November 10 | 0 | Breach of security | Medical | Computers in a linear accelerator facility were vandalized. The linear accelerator was not tampered with. A security assessment of the facility was undertaken by the licensee, recommendations were made, and an implementation plan was put in place. |
| WNSL-15 | November 11 | 0 | Release | Commercial | Wastewater streams were discharged from holding tanks. Subsequent analysis of a sample indicated that the total phosphorus (non-radioactive) released was slightly higher than limits. There were no adverse environmental effects. |
| 5402 | November 17 | 0 | Unplanned exposure | Industrial | A worker (member of the public) at a job site crossed a barrier during an industrial radiography exposure. Based on the position of the worker and their time in the area, there was no overexposure to the member of the public as a result of this event. |
| 5403 | November 19 | 0 | Breach of security | Medical | A treatment room containing prescribed equipment was left unsecured with the motion sensor disarmed. There was no actual attempted intrusion. |
| 5404 | November 20 | 0 | Damaged device | Industrial | A portable gauge was damaged by a snowplow on a construction site. Only the casing of the gauge was damaged. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of the event. |
| 5406 | November 23 | 0 | Transport – MVA | Commercial | A vehicle transporting excepted packages with trace amounts of technetium-99m was involved in a minor MVA. There was no damage to the packages. |
| 5413 | November 26 | 0 | Transport | Medical | Contamination was detected on the exterior of packages containing medical isotopes. The hands of one non-NEW were contaminated; however, there were no overexposures as a result of this event. |
| 5415 | December 1 | 0 | Contamination | Medical | A NEW’s wrist was contaminated with lutetium-177. A dose change request was submitted to add the dose to the NEW’s extremity dose record. There were no overexposures as a result of this event. |
| 5416 | December 1 | 0 | Damaged device | Industrial | A fixed gauge was found to be bent, causing it to malfunction. The gauge was removed from service for disposal. |
| 5418 | December 1 | 0 | Breach of security | Medical | During routine testing, the licensee’s security measures did not function as intended. There was no actual attempted intrusion. |
| 5421 | December 2 | 0 | Breach of security | Academic and research | An untrained cleaning staff member had access to a room with a self-shielded irradiator. Access has been removed. |
| 5484 | December 2 | 0 | Breach of security | Medical | A cyber-security event was reported at the licensed facility. |
| 5422 | December 6 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5423 | December 7 | 0 | Spill | Medical | A spill greater than 100 EQ of technetium-99m occurred. There was no skin contamination and there were no overexposures as a result of this event. |
| 5424 | December 7 | 0 | Damaged device | Industrial | The shutter handle of a fixed gauge became detached. The gauge will be replaced. There were no overexposures as a result of this event. |
| 5427 | December 8 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5431 | December 8 | 0 | Spill | Medical | A spill greater than 100 EQ of technetium-99m occurred. The technologist was contaminated; however, there were no overexposures as a result of this event. |
| 5429 | December 9 | 0 | Device malfunction | Industrial | The shutter of a fixed gauge was stuck in the open position. The shutter was repaired. There were no overexposures as a result of this event. |
| 5432 | December 9 | 0 | Damaged device | Industrial | A portable gauge was run over by a bulldozer on a construction site. The gauge was removed from service and sent for disposal. There was no loss of containment and no overexposures as a result of the event. |
| 5436 | December 14 | 0 | Damaged device | Industrial | A fixed gauge was found to be bent, causing it to malfunction. The gauge was removed from service for disposal. |
| 5440 | December 16 | 0 | Breach of security | Medical | An alarm was not set on a room that contains sealed sources. There was no actual attempted intrusion. |
| WNSL-16 | December 17 | 0 | Security | Commercial | Security-related event – Confidential |
| 5462 | December 21 | 0 | Transport – MVA | Industrial | A vehicle transporting a portable gauge was involved in an MVA. There was no damage to the portable gauge. |
| 5443 | December 24 | 0 | Damaged device | Industrial | A portable gauge was damaged when the corner banged against a concrete foundation. Only the casing was damaged. The gauge was removed from service and sent for repair. There was no loss of containment and no overexposures as a result of this event. |
| 5445 | December 28 | 0 | Device malfunction | Industrial | The shutter of a fixed gauge was fixed in the closed position. The gauge was removed from service for possible repair. There were no overexposures as a result of this event. |
Appendix F: Inspections conducted in 2021
| Inspection date | Licensee name | City | Province/State | Inspection type | Sector |
|---|---|---|---|---|---|
| 2021-01-06 | Stantec Consulting Ltd. | Laval | Quebec | Type II | Industrial |
| 2021-01-06 | Roxul Inc. | Grand Forks | British Columbia | Type II | Industrial |
| 2021-01-11 | UT Quality Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-01-12 | Cariboo Pulp & Paper Company | Quesnel | British Columbia | Type II | Industrial |
| 2021-01-13 | Bulkley Valley Engineering Services Ltd. | Smithers | British Columbia | Type II | Industrial |
| 2021-01-13 | Résoscan Inc. | Greenfield Park | Quebec | Type II | Medical |
| 2021-01-14 | Collège d’enseignement général et professsionnel de Trois-Rivières | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-01-14 | Steel Inspection & Testing Ltd. | St. Catharines | Ontario | Type II | Industrial |
| 2021-01-14 | Mills Memorial Hospital | Terrace | British Columbia | Type II | Medical |
| 2021-01-14 | Mills Memorial Hospital | Terrace | British Columbia | Type II | Medical |
| 2021-01-15 | Kubota Materials Canada Corporation | Orillia | Ontario | Type II | Industrial |
| 2021-01-18 | Canadian Light Source | Saskatoon | Saskatchewan | Type II | Academic and research |
| 2021-01-18 | Woodstock General Hospital | Woodstock | Ontario | Type II | Medical |
| 2021-01-19 | Ultratest N.D.T. Services (2010) Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-01-19 | Centre intégré de santé et de services sociaux de la Montérégie | Saint-Hyacinthe | Quebec | Type II | Medical |
| 2021-01-19 | Centre intégré de santé et de services sociaux de la Montérégie | Saint-Hyacinthe | Quebec | Type II | Medical |
| 2021-01-20 | Echo NDE Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-01-20 | Metalogic Inspection Services Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-01-20 | Teck Metals Ltd. | Trail | British Columbia | Type II | Industrial |
| 2021-01-20 | Rampure Radiology Associates Inc. | Windsor | Ontario | Type II | Medical |
| 2021-01-21 | FNX-INNOV Inc. | Longueuil | Quebec | Type II | Industrial |
| 2021-01-22 | ArcelorMittal Long Products Canada Real Estate Inc. | Contrecoeur | Quebec | Type II | Industrial |
| 2021-01-25 | 2540794 Ontario Inc. | Toronto | Ontario | Type II | Medical |
| 2021-01-26 | FB Nondestructive Examination Ltd. | Moose Jaw | Saskatchewan | Type II | Industrial |
| 2021-01-26 | Centre de santé et de services sociaux de Sept-Îles | Sept-Îles | Quebec | Type II | Medical |
| 2021-01-26 | Toronto West Cardiac and Medical Imaging Centre Ltd. | North York | Ontario | Type II | Medical |
| 2021-01-26 | Centre intégré de santé et de services sociaux de Lanaudière | Saint-Charles-Borromée | Quebec | Type II | Medical |
| 2021-01-26 | Centre de santé et de services sociaux de Sept-Îles | Sept-Îles | Quebec | Type II | Medical |
| 2021-01-26 | Centre intégré de santé et de services sociaux de Lanaudière | Saint-Charles-Borromée | Quebec | Type II | Medical |
| 2021-01-27 | West-Can Inspection Ltd. | Sunnyside | Manitoba | Type II | Industrial |
| 2021-01-27 | Humber River Hospital | Toronto | Ontario | Type II | Medical |
| 2021-01-27 | Humber River Hospital | Toronto | Ontario | Type II | Medical |
| 2021-01-28 | Royal Military College of Canada | Kingston | Ontario | Type II | Academic and research |
| 2021-01-28 | Dexter Construction Company Limited | Waverley | Nova Scotia | Type II | Industrial |
| 2021-01-28 | Union Street Geotechnical Ltd. | Red Deer | Alberta | Type II | Industrial |
| 2021-01-28 | Northern Alberta Institute of Technology | Edmonton | Alberta | Type II | Industrial |
| 2021-01-28 | Silicium Québec Commandité Inc. | Bécancour | Quebec | Type II | Industrial |
| 2021-01-28 | Teck Coal Limited | Sparwood | British Columbia | Type II | Industrial |
| 2021-01-28 | Teck Coal Limited | Sparwood | British Columbia | Type II | Industrial |
| 2021-01-29 | Elander Inspections Ltd. | Richmond | British Columbia | Type II | Industrial |
| 2021-02-01 | Clear Image Inspection Ltd. | Bentley | Alberta | Type II | Industrial |
| 2021-02-02 | Nordion (Canada) Inc. | Kanata | Ontario | Type I | Commercial |
| 2021-02-02 | RTD Quality Services Inc. | Victoria | British Columbia | Type II | Industrial |
| 2021-02-02 | Hunt Inspection Ltd. | Lacombe | Alberta | Type II | Industrial |
| 2021-02-02 | Custom Fabricators & Machinists Limited / Fabricants et Machinistes Industrielle Limitée | Saint John | New Brunswick | Type II | Industrial |
| 2021-02-03 | Interior Health Authority | Cranbrook | British Columbia | Type II | Medical |
| 2021-02-03 | Centre intégré universitaire de santé et de services sociaux | Montréal | Quebec | Type II | Medical |
| 2021-02-03 | Interior Health Authority | Cranbrook | British Columbia | Type II | Medical |
| 2021-02-03 | Centre intégré universitaire de santé et de services sociaux du Nord-de-l’Île-de-Montréal | Montréal | Quebec | Type II | Medical |
| 2021-02-04 | Municipal Enterprises Limited | Bedford | Nova Scotia | Type II | Industrial |
| 2021-02-05 | Women's College Hospital | Toronto | Ontario | Type II | Medical |
| 2021-02-05 | Chatham-Kent Health Alliance | Chatham | Ontario | Type II | Medical |
| 2021-02-05 | Women's College Hospital | Toronto | Ontario | Type II | Medical |
| 2021-02-05 | Chatham-Kent Health Alliance | Chatham | Ontario | Type II | Medical |
| 2021-02-09 | General Dynamics | Repentigny | Quebec | Type II | Industrial |
| 2021-02-09 | Q Test Inspection Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-02-09 | Merivale Medical Imagining Inc. | Nepean | Ontario | Type II | Medical |
| 2021-02-09 | McConnell Brain Imaging Centre | Montréal | Quebec | Type II | Medical |
| 2021-02-09 | McConnell Brain Imaging Centre | Montréal | Quebec | Type II | Medical |
| 2021-02-10 | Peterson Contracting Ltd. | Williams Lake | British Columbia | Type II | Industrial |
| 2021-02-10 | Perfection Inspection Limited | Cambridge | Ontario | Type II | Industrial |
| 2021-02-10 | Unique Detection Services Limited | Cambridge | Ontario | Type II | Industrial |
| 2021-02-10 | Rain Carbon Canada Inc. | Hamilton | Ontario | Type II | Industrial |
| 2021-02-10 | Schlumberger Canada Limited | Nisku | Alberta | Type II | Industrial |
| 2021-02-10 | Izaak Walton Killam Health Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-02-10 | Ottawa Cardiovascular Centre – Orleans Inc. | Ottawa | Ontario | Type II | Medical |
| 2021-02-11 | Mississauga Metals and Alloys | Brantford | Ontario | Type II | Commercial |
| 2021-02-11 | Nelson's Welding Inspection Limited | Drayton Valley | Alberta | Type II | Industrial |
| 2021-02-11 | Stratford General Hospital | Stratford | Ontario | Type II | Medical |
| 2021-02-11 | Stratford General Hospital | Stratford | Ontario | Type II | Medical |
| 2021-02-12 | Hôpital Montfort | Ottawa | Ontario | Type II | Medical |
| 2021-02-13 | Med-Scan Ultrasound Services Ltd. | Maple | Ontario | Type II | Medical |
| 2021-02-15 | Focus NDTIS Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-02-17 | Fluid Projects Consulting Inc. | Calgary | Alberta | Type II | Industrial |
| 2021-02-17 | Syncrude Canada Ltd. | Fort McMurray | Alberta | Type II | Industrial |
| 2021-02-18 | Centre universitaire de santé McGill / McGill University Health Centre | Montréal | Quebec | Type II | Medical |
| 2021-02-19 | Commandité Stadacona WB Ltée | Québec City | Quebec | Type II | Industrial |
| 2021-02-22 | Thunder Bay Regional Health Sciences Centre | Thunder Bay | Ontario | Type I | Medical |
| 2021-02-23 | Ontario Power Generation | Toronto | Ontario | Type II | Academic and research |
| 2021-02-23 | Simon Fraser University | Burnaby | British Columbia | Type II | Academic and research |
| 2021-02-23 | Centre hospitalier Sainte-Croix | Drummondville | Quebec | Type II | Medical |
| 2021-02-23 | Centre hospitalier Sainte-Croix | Drummondville | Quebec | Type II | Medical |
| 2021-02-24 | Gregson Holdings Ltd. | Nanaimo | British Columbia | Type II | Industrial |
| 2021-02-24 | Les Laboratoires d'Essais Mequaltech Inc. | Lévis | Quebec | Type II | Industrial |
| 2021-02-24 | Institut de Cardiologie de Montréal | Montréal | Quebec | Type II | Medical |
| 2021-02-24 | Winnipeg Regional Health Authority | Winnipeg | Manitoba | Type II | Medical |
| 2021-02-24 | Initio Medical Group Inc. | Burnaby | British Columbia | Type II | Medical |
| 2021-02-24 | Centre intégré universitaire de santé et de services sociaux | Victoriaville | Quebec | Type II | Medical |
| 2021-02-24 | Winnipeg Regional Health Authority | Winnipeg | Manitoba | Type II | Medical |
| 2021-02-24 | Centre intégré universitaire de santé et de services sociaux | Victoriaville | Quebec | Type II | Medical |
| 2021-02-24 | Institut de Cardiologie de Montréal | Montréal | Quebec | Type II | Medical |
| 2021-02-25 | Thurber Engineering Ltd. | Victoria | British Columbia | Type II | Industrial |
| 2021-02-25 | Labcan (1989) Ltée | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-02-25 | Thermo Design Engineering Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-02-25 | Regional Health Authority A | Bathurst | New Brunswick | Type II | Medical |
| 2021-02-25 | York X-Ray Management Limited O/A York Radiology Consultants | Willowdale | Ontario | Type II | Medical |
| 2021-03-01 | Simon Fraser University | Burnaby | British Columbia | Type II | Academic and research |
| 2021-03-01 | D.L.H Medical Inc. | Brampton | Ontario | Type II | Medical |
| 2021-03-02 | Queensway Carleton Hospital | Nepean | Ontario | Type II | Medical |
| 2021-03-03 | Semm Logging Inc. | Mississauga | Ontario | Type II | Industrial |
| 2021-03-03 | Oak Ridges Medical Diagnostic Imaging Inc. | Richmond Hill | Ontario | Type II | Medical |
| 2021-03-03 | Clinique Radiologique de la Capitale Inc. | Québec City | Quebec | Type II | Medical |
| 2021-03-04 | Foothills Radiography & Inspection Services Ltd. | Edson | Alberta | Type II | Industrial |
| 2021-03-04 | Brant Community Healthcare System | Brantford | Ontario | Type II | Medical |
| 2021-03-04 | Brant Community Healthcare System | Brantford | Ontario | Type II | Medical |
| 2021-03-05 | Ontario Power Generation Inc. | Bowmanville | Ontario | Type II | Industrial |
| 2021-03-08 | Dixie X-Ray Associates Limited | Woodbridge | Ontario | Type II | Medical |
| 2021-03-09 | E.F. Monk Holdings Limited | Dartmouth | Nova Scotia | Type II | Industrial |
| 2021-03-09 | WRHA Grace Hospital Site | Winnipeg | Manitoba | Type II | Medical |
| 2021-03-10 | Suncor Energy Inc. | Fort McMurray | Alberta | Type II | Industrial |
| 2021-03-10 | Northumberland Hills Hospital | Cobourg | Ontario | Type II | Medical |
| 2021-03-11 | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | Ontario | Type II | Commercial |
| 2021-03-11 | Catalyst Paper Corporation | Port Alberni | British Columbia | Type II | Industrial |
| 2021-03-11 | Reliance OFS Canada Ltd. | Estevan | Saskatchewan | Type II | Industrial |
| 2021-03-11 | Centre intégré de santé et de services sociaux de la Montérégie | Salaberry-de-Valleyfield | Quebec | Type II | Medical |
| 2021-03-11 | Centre intégré de santé et de services sociaux de la Montérégie | Salaberry-de-Valleyfield | Quebec | Type II | Medical |
| 2021-03-12 | Flatiron Construction Canada Limited | Lillooet | British Columbia | Type II | Industrial |
| 2021-03-15 | Alberta Health Services | Calgary | Alberta | Type I | Medical |
| 2021-03-15 | Victoria General Hospital | Winnipeg | Manitoba | Type II | Medical |
| 2021-03-19 | Toronto Cardiac Diagnostics Inc. | North York | Ontario | Type II | Medical |
| 2021-03-22 | Candu Energy Inc. | Mississauga | Ontario | Type II | Commercial |
| 2021-03-22 | Suncor Energy Inc./ Suncor Énergie Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-03-23 | 1068648 B.C. Ltd. | Terrace | British Columbia | Type II | Industrial |
| 2021-03-24 | Wood Canada Limited / Wood Canada Limitée | Terrace | British Columbia | Type II | Industrial |
| 2021-03-24 | Canadoil Forge Ltée / Canadoil Forge Ltd. | Bécancour | Quebec | Type II | Industrial |
| 2021-03-24 | NHS – St. Catharines Site | St. Catharines | Ontario | Type II | Medical |
| 2021-03-24 | Di-Med Services Limited | Aurora | Ontario | Type II | Medical |
| 2021-03-24 | NHS – St. Catharines Site | St. Catharines | Ontario | Type II | Medical |
| 2021-03-25 | Corcare Nuclear Medicine Inc. | Toronto | Ontario | Type II | Medical |
| 2021-03-25 | 2345171 Ontario Inc. | Guelph | Ontario | Type II | Medical |
| 2021-03-25 | MyHealth Partners Inc. | London | Ontario | Type II | Medical |
| 2021-03-30 | Institut universitaire de cardiologie et de pneumologie de Québec | Sainte-Foy | Quebec | Type II | Medical |
| 2021-03-30 | Centre intégré de santé et de services sociaux de Lanaudière | Lachenaie | Quebec | Type II | Medical |
| 2021-03-30 | Institut universitaire de cardiologie et de pneumologie de Québec | Sainte-Foy | Quebec | Type II | Medical |
| 2021-03-30 | Centre intégré de santé et de services sociaux de Lanaudière | Lachenaie | Quebec | Type II | Medical |
| 2021-03-31 | Goldcorp Canada Ltd. | Houston | British Columbia | Type II | Industrial |
| 2021-04-01 | Delwisch Developments Ltd. | Smithers | British Columbia | Type II | Industrial |
| 2021-04-06 | Cascades Sonoco Inc. | Kingsey Falls | Quebec | Type II | Industrial |
| 2021-04-06 | Cancer Care Manitoba | Winnipeg | Manitoba | Type II | Medical |
| 2021-04-06 | Winnipeg Regional Health Authority | Winnipeg | Manitoba | Type II | Medical |
| 2021-04-06 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Medical |
| 2021-04-06 | QEII Health Sciences Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-04-08 | Saskatchewan Health Authority | Saskatoon | Saskatchewan | Type II | Medical |
| 2021-04-09 | FP Innovations | Vancouver | British Columbia | Type II | Industrial |
| 2021-04-09 | The Ottawa Hospital | Ottawa | Ontario | Type II | Medical |
| 2021-04-09 | Regina Qu'Appelle Health Region | Regina | Saskatchewan | Type II | Medical |
| 2021-04-09 | Regina Qu'Appelle Health Region | Regina | Saskatchewan | Type II | Medical |
| 2021-04-09 | The Ottawa Hospital | Ottawa | Ontario | Type II | Medical |
| 2021-04-12 | AM Inspection Limited | Weyburn | Saskatchewan | Type II | Industrial |
| 2021-04-13 | Bonnechere Excavating Inc. | Renfrew | Ontario | Type II | Industrial |
| 2021-04-13 | Paragon Wireline Services Ltd. | Calmar | Alberta | Type II | Industrial |
| 2021-04-13 | Cambridge Memorial Hospital | Cambridge | Ontario | Type II | Medical |
| 2021-04-14 | Regional Municipality of Durham | Whitby | Ontario | Type II | Industrial |
| 2021-04-14 | Emil Anderson Construction Co. Ltd. | Vancouver Island | British Columbia | Type II | Industrial |
| 2021-04-14 | H & H Construction Inc. | Petawawa | Ontario | Type II | Industrial |
| 2021-04-14 | Louis Dreyfus Company Canada ULC | Yorkton | Saskatchewan | Type II | Industrial |
| 2021-04-14 | Centre hospitalier universitaire Sainte-Justine | Montréal | Quebec | Type II | Medical |
| 2021-04-14 | Centre de santé et de services sociaux du sud-ouest-Verdun | Verdun | Quebec | Type II | Medical |
| 2021-04-14 | Centre hospitalier universitaire Sainte-Justine | Montréal | Quebec | Type II | Medical |
| 2021-04-14 | Centre de santé et de services sociaux du sud-ouest-Verdun | Verdun | Quebec | Type II | Medical |
| 2021-04-14 | Centre hospitalier universitaire Sainte-Justine | Montréal | Quebec | Type II | Medical |
| 2021-04-19 | Mahlo America, Inc. | Montréal | Quebec | Type II | Commercial |
| 2021-04-19 | Hôpital Maisonneuve-Rosemont | Montréal | Quebec | Type I | Medical |
| 2021-04-20 | TISI Canada Inc. | Kitchener | Ontario | Type II | Industrial |
| 2021-04-21 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Longueuil | Quebec | Type II | Medical |
| 2021-04-21 | Centre intégré de santé et de services sociaux de la Montérégie-Est | Longueuil | Quebec | Type II | Medical |
| 2021-04-22 | Huckleberry Mines Ltd. | Houston | British Columbia | Type II | Industrial |
| 2021-04-22 | Mistras Services Inc. | Oakville | Ontario | Type II | Industrial |
| 2021-04-22 | Huckleberry Mines Ltd. | Houston | British Columbia | Type II | Industrial |
| 2021-04-26 | Regional Health Authority B | Saint John | New Brunswick | Type II | Medical |
| 2021-04-27 | Suncor Energy Inc./Suncor Énergie Inc. | Sarnia | Ontario | Type II | Industrial |
| 2021-04-28 | Service New Brunswick | Saint John | New Brunswick | Type II | Commercial |
| 2021-04-28 | Pro-Test Professional Testing & Inspection Co. Ltd. | Winnipeg | Manitoba | Type II | Industrial |
| 2021-04-28 | Samuel, Son & Co. Limited | Stoney Creek | Ontario | Type II | Industrial |
| 2021-04-28 | Samuel, Son & Co. Limited | Burlington | Ontario | Type II | Industrial |
| 2021-04-28 | Samuel, Son & Co. Limited | Winnipeg | Manitoba | Type II | Industrial |
| 2021-04-28 | Centre intégré de santé et de services sociaux de l’Abitibi-Témiscamingue | Rouyn-Noranda | Quebec | Type II | Medical |
| 2021-04-29 | Vanko Analytics Limited | Edmonton | Alberta | Type II | Commercial |
| 2021-04-29 | CT & Associates Engineering Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-04-29 | Lavis Contracting Co. Limited | Clinton | Ontario | Type II | Industrial |
| 2021-04-29 | E.I. DuPont Canada Company | Kingston | Ontario | Type II | Industrial |
| 2021-04-30 | The University Hospital of Northern British Columbia | Prince George | British Columbia | Type II | Medical |
| 2021-04-30 | The University Hospital of Northern British Columbia | Prince George | British Columbia | Type II | Medical |
| 2021-05-05 | Vancouver Island Health Authority | Nanaimo | British Columbia | Type II | Medical |
| 2021-05-05 | Centre intégré universitaire de santé et de services sociaux | Montréal | Quebec | Type II | Medical |
| 2021-05-05 | Vancouver Island Health Authority | Nanaimo | British Columbia | Type II | Medical |
| 2021-05-05 | Centre intégré universitaire de santé et de services sociaux | Montréal | Quebec | Type II | Medical |
| 2021-05-06 | Wood Canada Limited / Wood Canada Limitée | Prince George | British Columbia | Type II | Industrial |
| 2021-05-06 | Horton CBI, Limited | Sturgeon County | Alberta | Type II | Industrial |
| 2021-05-11 | Parkland Geotechnical Consulting Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-05-11 | Oshanek Inspection Services (1972) Ltd. | Fox Creek | Alberta | Type II | Industrial |
| 2021-05-11 | Oshanek Inspection Services (1972) Ltd. | Grande Prairie | Alberta | Type II | Industrial |
| 2021-05-12 | Resource Management International Inc. | Lashburn | Saskatchewan | Type II | Industrial |
| 2021-05-12 | Northern Alberta Institute of Technology | Edmonton | Alberta | Type II | Industrial |
| 2021-05-12 | Air Transat A.T. Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-05-19 | Centre intégré universitaire de santé et de services sociaux | Montréal | Quebec | Type II | Medical |
| 2021-05-19 | Centre intégré universitaire de santé et de services sociaux | Montréal | Quebec | Type II | Medical |
| 2021-05-21 | De Beers Canada Inc. | Yellowknife | Northwest Territories | Type II | Industrial |
| 2021-05-21 | De Beers Canada Inc. | Yellowknife | Northwest Territories | Type II | Industrial |
| 2021-05-21 | Catalyst Paper Corporation | Crofton | British Columbia | Type II | Industrial |
| 2021-05-25 | Université de Montréal | Montréal | Quebec | Type II | Academic and research |
| 2021-05-25 | Kodiak Nondestructive Testing Services Ltd. | Nanaimo | British Columbia | Type II | Industrial |
| 2021-05-25 | Tracerco Radioactive Diagnostic Services Canada, Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-05-26 | Nucléom Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-05-27 | École Polytechnique de Montréal | Montréal | Quebec | Type II | Academic and research |
| 2021-05-27 | Canadian Inspection Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-05-27 | Atlantic Coated Papers Ltd. / Papiers Couchés d'Atlantic Ltée | Whitby | Ontario | Type II | Industrial |
| 2021-05-28 | RTD Quality Services Inc. | Burlington | Ontario | Type II | Industrial |
| 2021-05-28 | The Minute Maid Company Canada Inc./ | Peterborough | Ontario | Type II | Industrial |
| 2021-05-31 | CISSS de Laval | Laval | Quebec | Type I | Medical |
| 2021-06-01 | Niagara Health System | St. Catharines | Ontario | Type I | Medical |
| 2021-06-01 | Alberta Health Services | Edmonton | Alberta | Type II | Medical |
| 2021-06-02 | RTD Quality Services Inc. | Surrey | British Columbia | Type II | Industrial |
| 2021-06-02 | Alberta Health Services | Edmonton | Alberta | Type II | Medical |
| 2021-06-03 | B. J. Halow & Son Constructors Ltd. | Rosslyn | Ontario | Type II | Industrial |
| 2021-06-03 | Kam Tech Quality Management Inc. | Kamloops | British Columbia | Type II | Industrial |
| 2021-06-04 | TISI Canada Inc. | Slave Lake | Alberta | Type II | Industrial |
| 2021-06-06 | Centre for Addiction and Mental Health | Toronto | Ontario | Type I | Commercial |
| 2021-06-07 | Health Sciences North | Sudbury | Ontario | Type I | Medical |
| 2021-06-08 | Université du Québec à Rimouski | Rimouski | Quebec | Type II | Academic and research |
| 2021-06-08 | Université du Québec à Rimouski | Rimouski | Quebec | Type II | Academic and research |
| 2021-06-08 | Université du Québec à Rimouski | Rimouski | Quebec | Type II | Academic and research |
| 2021-06-08 | Université du Québec à Rimouski | Rimouski | Quebec | Type II | Industrial |
| 2021-06-09 | Pinchin Ltd. | Waterloo | Ontario | Type II | Industrial |
| 2021-06-09 | IRISNDT Corp. | Edmonton | Alberta | Type II | Industrial |
| 2021-06-09 | 2376440 Ontario Inc. | Sudbury | Ontario | Type II | Medical |
| 2021-06-09 | Guelph General Hospital | Guelph | Ontario | Type II | Medical |
| 2021-06-10 | 9372-2619 Québec inc. | Alma | Quebec | Type II | Industrial |
| 2021-06-11 | McElhanney Ltd. | Prince George | British Columbia | Type II | Industrial |
| 2021-06-11 | Suncor Energy Inc./Suncor Énergie Inc. | Sarnia | Ontario | Type II | Industrial |
| 2021-06-14 | Frontop Engineering Limited | Markham | Ontario | Type II | Industrial |
| 2021-06-14 | Trenergy Inc. | St. Catharines | Ontario | Type II | Industrial |
| 2021-06-16 | Alberta Power (2000) Ltd. | Forestburg | Alberta | Type II | Industrial |
| 2021-06-17 | New Gold Inc. | Kamloops | British Columbia | Type II | Industrial |
| 2021-06-17 | Acuren Inc. | Fort Saskatchewan | Alberta | Type II | Industrial |
| 2021-06-17 | New Gold Inc. | Kamloops | British Columbia | Type II | Industrial |
| 2021-06-18 | Terraspec Engineering Inc. | Peterborough | Ontario | Type II | Industrial |
| 2021-06-21 | Eastern Regional Health Authority | St. John's | Newfoundland and Labrador | Type II | Commercial |
| 2021-06-21 | Les Inspections Thermetco Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-06-22 | Intratech Engineering Laboratories Ltd. | Scarborough | Ontario | Type II | Industrial |
| 2021-06-22 | Les Inspections Thermetco Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-06-23 | St. Mary's General Hospital | Kitchener | Ontario | Type II | Medical |
| 2021-06-23 | St. Mary's General Hospital | Kitchener | Ontario | Type II | Medical |
| 2021-06-25 | Hôpital Régional d'Edmundston | Edmundston | New Brunswick | Type II | Medical |
| 2021-06-25 | Régie Régionale de la Santé A, Bureau 600 | Edmundston | New Brunswick | Type II | Medical |
| 2021-06-28 | Glassine Canada Inc. | Québec City | Quebec | Type II | Industrial |
| 2021-06-29 | Capital Paving Inc. | Puslinch | Ontario | Type II | Industrial |
| 2021-06-29 | MDG Contracting Services Inc. | Likely | British Columbia | Type II | Industrial |
| 2021-06-29 | Eastern Regional Health Authority | St. John's | Newfoundland and Labrador | Type II | Medical |
| 2021-06-29 | Eastern Regional Health Authority | St. John's | Newfoundland and Labrador | Type II | Medical |
| 2021-06-30 | Terra International (Canada) Inc. | Courtright | Ontario | Type II | Industrial |
| 2021-07-05 | Hartstone Inc. | Olds | Alberta | Type II | Industrial |
| 2021-07-05 | Meadow Lake Mechanical Pulp Ltd. | Meadow Lake | Saskatchewan | Type II | Industrial |
| 2021-07-05 | Alberta Health Services | Red Deer | Alberta | Type I | Medical |
| 2021-07-06 | Abraflex (2004) Ltd. | Paisley | Ontario | Type II | Commercial |
| 2021-07-07 | Terra International (Canada) Inc. | Courtright | Ontario | Type II | Industrial |
| 2021-07-07 | Orillia Soldier's Memorial Hospital | Orillia | Ontario | Type II | Medical |
| 2021-07-09 | Forward Engineering & Associates Inc. | Toronto | Ontario | Type II | Industrial |
| 2021-07-09 | Taylor Geotechnical Ltd. | Canmore | Alberta | Type II | Industrial |
| 2021-07-12 | Accuray Incorporated | Sunnyvale | California | Type I | Commercial |
| 2021-07-12 | XE Inspection Inc. | Fort McMurray | Alberta | Type II | Industrial |
| 2021-07-12 | R.W. Tomlinson Limited | Ottawa | Ontario | Type II | Industrial |
| 2021-07-13 | Golder Associates Ltd. | Cambridge | Ontario | Type II | Industrial |
| 2021-07-13 | Chung & Vander Doelen Engineering Ltd. | Kitchener | Ontario | Type II | Industrial |
| 2021-07-13 | Steed and Evans Limited | St. Jacobs | Ontario | Type II | Industrial |
| 2021-07-13 | Pinchin Ltd. | Waterloo | Ontario | Type II | Industrial |
| 2021-07-13 | AM Inspection Limited | Stettler | Alberta | Type II | Industrial |
| 2021-07-13 | AM Inspection Limited | Stettler | Alberta | Type II | Industrial |
| 2021-07-14 | Q Test Inspection Ltd. | Sylvan Lake | Alberta | Type II | Industrial |
| 2021-07-14 | TISI Canada Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-07-14 | TISI Canada Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-07-15 | Terracon Geotechnique Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-07-15 | Independent Materials Testing Services Ltd. | Regina | Saskatchewan | Type II | Industrial |
| 2021-07-15 | PRI Engineering Corp. | Lindsay | Ontario | Type II | Industrial |
| 2021-07-15 | Mistras Canada, Inc. | Sherwood Park | Alberta | Type II | Industrial |
| 2021-07-15 | Thunder Bay Regional Health Sciences Centre | Thunder Bay | Ontario | Type II | Medical |
| 2021-07-16 | Intratech Engineering Laboratories Ltd. | Scarborough | Ontario | Type II | Industrial |
| 2021-07-16 | Toronto Inspection Ltd. | Markham | Ontario | Type II | Industrial |
| 2021-07-16 | Interface Testing Services Inc. | Sarnia | Ontario | Type II | Industrial |
| 2021-07-19 | C.B. Non-Destructive Testing Ltd | Oakville | Ontario | Type II | Industrial |
| 2021-07-20 | Golder Associates Ltd. | Barrie | Ontario | Type II | Industrial |
| 2021-07-20 | Soil Engineers Ltd. | Richmond Hill | Ontario | Type II | Industrial |
| 2021-07-20 | Davroc Testing Laboratories Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-07-20 | H. Manalo Consulting | Winnipeg | Manitoba | Type II | Industrial |
| 2021-07-20 | Peto MacCallum Ltd. | Barrie | Ontario | Type II | Industrial |
| 2021-07-20 | BDT Engineering Ltd | Lethbridge | Alberta | Type II | Industrial |
| 2021-07-20 | WSP Canada Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-07-20 | WSP Canada Inc. | Barrie | Ontario | Type II | Industrial |
| 2021-07-20 | EXP Services Inc. / Les Services EXP Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-07-20 | EXP Services Inc. / Les Services EXP Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-07-20 | Roseke Engineering Ltd. | Lethbridge | Alberta | Type II | Industrial |
| 2021-07-21 | AM Inspection Limited | Stettler | Alberta | Type II | Industrial |
| 2021-07-21 | AM Inspection Limited | Stettler | Alberta | Type II | Industrial |
| 2021-07-21 | Building Products of Canada Corp. | La Salle | Quebec | Type II | Industrial |
| 2021-07-22 | Smith Dow & Associates Ltd. | Red Deer | Alberta | Type II | Industrial |
| 2021-07-22 | 9395-8049 Québec inc. | Saint-Jérôme | Quebec | Type II | Industrial |
| 2021-07-22 | 9395-8049 Québec inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-07-22 | Candec Consultants Ltd. | Richmond Hill | Ontario | Type II | Industrial |
| 2021-07-22 | Sola Engineering Inc. | Vaughan | Ontario | Type II | Industrial |
| 2021-07-22 | DS Consultants Ltd. | Vaughan | Ontario | Type II | Industrial |
| 2021-07-22 | FNX-INNOV Inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-07-22 | Les Laboratoires d'Essais Mequaltech Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-07-23 | MPE Engineering Ltd. | Lethbridge | Alberta | Type II | Industrial |
| 2021-07-23 | Tetra Tech Canada Inc. | Lethbridge | Alberta | Type II | Industrial |
| 2021-07-23 | Peter Kiewit Sons ULC | Calgary | Alberta | Type II | Industrial |
| 2021-07-23 | LN Land Development Technologies Inc. | Lacombe | Alberta | Type II | Industrial |
| 2021-07-27 | Chung & Vander Doelen Engineering Ltd. | Kitchener | Ontario | Type II | Industrial |
| 2021-07-27 | M.C.P.D. Consultants Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-07-27 | Coco Paving Inc. | Hamilton | Ontario | Type II | Industrial |
| 2021-07-27 | Engtec Consulting Inc. | Mississauga | Ontario | Type II | Industrial |
| 2021-07-27 | Soil-Mat Engineers & Consultants Ltd. | Hamilton | Ontario | Type II | Industrial |
| 2021-07-27 | Uni-Tech Inspections Services Ltd. | South Glengarry | Ontario | Type II | Industrial |
| 2021-07-27 | EnviroGeotech Consulting Inc. | Medicine Hat | Alberta | Type II | Industrial |
| 2021-07-28 | Queen's University | Kingston | Ontario | Type II | Academic and research |
| 2021-07-28 | Uni-Vert Tech Inc. | Sainte-Marcelline-de-Kildare | Quebec | Type II | Commercial |
| 2021-07-28 | Uni-Vert Tech Inc. | Sainte-Marcelline-de-Kildare | Quebec | Type II | Commercial |
| 2021-07-28 | Thurber Engineering Ltd. | Oakville | Ontario | Type II | Industrial |
| 2021-07-28 | Englobe Corp. | Laval | Quebec | Type II | Industrial |
| 2021-07-28 | GeoPacific Consultants Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-07-28 | Stantec Consulting Ltd. | Waterloo | Ontario | Type II | Industrial |
| 2021-07-28 | SNC-Lavalin GEM Québec Inc. | Laval | Quebec | Type II | Industrial |
| 2021-07-28 | Lone Pine Geotechnical Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-07-28 | Lone Pine Geotechnical Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-07-28 | EXP Services Inc. / Les Services EXP Inc. | Halifax | Nova Scotia | Type II | Industrial |
| 2021-07-28 | Watt Consulting Group Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-07-28 | LN Land Development Technologies Inc. | Lacombe | Alberta | Type II | Industrial |
| 2021-07-29 | Hydro-Québec | Varennes | Quebec | Type II | Industrial |
| 2021-07-30 | Aecon Construction and Materials Limited | Caledon | Ontario | Type II | Industrial |
| 2021-07-30 | Highway Construction Inspection Ontario Inc. | Barrie | Ontario | Type II | Industrial |
| 2021-07-30 | Miller Paving Limited | Markham | Ontario | Type II | Industrial |
| 2021-07-30 | Canadian Blood Services | Ottawa | Ontario | Type II | Medical |
| 2021-08-03 | Stantec Consulting Ltd. | Port Hawkesbury | Nova Scotia | Type II | Industrial |
| 2021-08-03 | Watt Consulting Group Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-08-04 | Steed and Evans Limited | St. Jacobs | Ontario | Type II | Industrial |
| 2021-08-05 | Global Engineering & Testing Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-08-05 | Groupe ABS Inc. | Vaudreuil | Quebec | Type II | Industrial |
| 2021-08-05 | Tetra Tech Canada Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-08-09 | Stuart Hunt & Associates Ltd. | Mississauga | Ontario | Type II | Commercial |
| 2021-08-10 | Moncrief Construction Limited | Kenora | Ontario | Type II | Industrial |
| 2021-08-10 | Mistras Canada, Inc. | Red Deer | Alberta | Type II | Industrial |
| 2021-08-11 | Mahlo America, Inc. | Bolton-Est | Quebec | Type II | Commercial |
| 2021-08-11 | Ciment Québec | Saint-Basile | Quebec | Type II | Industrial |
| 2021-08-11 | Edward Wong & Associates Inc. | Markham | Ontario | Type II | Industrial |
| 2021-08-11 | Edward Wong & Associates Inc. | Markham | Ontario | Type II | Industrial |
| 2021-08-12 | Watt Consulting Group Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-08-12 | NWP Industries General Partner Ltd.. | Crossfield | Alberta | Type II | Industrial |
| 2021-08-13 | PNJ Engineering Inc. | Vaughan | Ontario | Type II | Industrial |
| 2021-08-13 | LAW Inspection Services Inc. | Lethbridge | Alberta | Type II | Industrial |
| 2021-08-16 | Atomic NDT Ltd. | Sylvan Lake | Alberta | Type II | Industrial |
| 2021-08-16 | Buffalo Inspection Services (2005) Inc. | Camrose | Alberta | Type II | Industrial |
| 2021-08-18 | Spectrum NDT Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-08-19 | Hartstone Inc. | Olds | Alberta | Type II | Industrial |
| 2021-08-20 | Cornwall Gravel Company Limited | Cornwall | Ontario | Type II | Industrial |
| 2021-08-20 | Davroc Testing Laboratories Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-08-20 | Englobe Corp. | Toronto | Ontario | Type II | Industrial |
| 2021-08-21 | Acuren Inc. | Cantley | Quebec | Type II | Industrial |
| 2021-08-23 | Thunder Bay Regional Health Sciences Centre | Thunder Bay | Ontario | Type I | Commercial |
| 2021-08-23 | Candec Consultants Ltd. | Richmond Hill | Ontario | Type II | Industrial |
| 2021-08-24 | Seymour Pacific Developments Ltd. | Winnipeg | Manitoba | Type II | Industrial |
| 2021-08-24 | Gamma-Tech Inspection Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-08-25 | Sunnybrook Health Sciences Centre | Toronto | Ontario | Type II | Commercial |
| 2021-08-25 | Morey Associates Limited | Kemptville | Ontario | Type II | Industrial |
| 2021-08-26 | 8418748 Canada Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-08-26 | Louis W. Bray Construction Limited | Vars | Ontario | Type II | Industrial |
| 2021-08-26 | Pavages Multipro inc. | Terrebonne | Quebec | Type II | Industrial |
| 2021-08-26 | 9139-6903 Québec Inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-08-26 | Les entreprises Rolland inc. | Saint-Jérôme | Quebec | Type II | Industrial |
| 2021-08-27 | Solroc Inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-08-27 | Bonnechere Excavating Inc. | Renfrew | Ontario | Type II | Industrial |
| 2021-08-27 | H & H Construction Inc. | Petawawa | Ontario | Type II | Industrial |
| 2021-08-27 | Nelson River Construction Inc. | Winnipeg | Manitoba | Type II | Industrial |
| 2021-08-30 | Alberta Health Services | Lethbridge | Alberta | Type II | Medical |
| 2021-08-31 | Clunie Consulting Engineers Ltd. | Prince Albert | Saskatchewan | Type II | Industrial |
| 2021-08-31 | Higher Ground Consulting Inc. | Calgary | Alberta | Type II | Industrial |
| 2021-08-31 | Groupe CRH Canada Inc. / CRH Canada Group Inc. | Laval | Quebec | Type II | Industrial |
| 2021-08-31 | Soleno Textiles Techniques Inc. | Laval | Quebec | Type II | Industrial |
| 2021-09-01 | District Municipality of Muskoka | Bracebridge | Ontario | Type II | Industrial |
| 2021-09-01 | Fowler Construction Company Ltd. | Bracebridge | Ontario | Type II | Industrial |
| 2021-09-01 | 9395-8049 Quebec inc. | Repentigny | Quebec | Type II | Industrial |
| 2021-09-01 | SNC-Lavalin GEM Québec Inc. | Laval | Quebec | Type II | Industrial |
| 2021-09-01 | IRISNDT Corp. | Calgary | Alberta | Type II | Industrial |
| 2021-09-01 | Titan Non-Destructive Examination Services Ltd. | Didsbury | Alberta | Type II | Industrial |
| 2021-09-02 | Greenwood Paving (Pembroke) Ltd. | Pembroke | Ontario | Type II | Industrial |
| 2021-09-02 | Thunder Bay Regional Health Sciences Centre | Thunder Bay | Ontario | Type II | Medical |
| 2021-09-03 | Voltage Wireline Inc. | Lacombe | Alberta | Type II | Industrial |
| 2021-09-06 | Pinter & Associates Ltd. | Saskatoon | Saskatchewan | Type II | Industrial |
| 2021-09-06 | Pinter & Associates Ltd. | Regina | Saskatchewan | Type II | Industrial |
| 2021-09-08 | University of Ottawa | Ottawa | Ontario | Type II | Academic and research |
| 2021-09-08 | McClymont and Rak Engineers Inc. | Vaughan | Ontario | Type II | Industrial |
| 2021-09-08 | Landtek Limited | Hamilton | Ontario | Type II | Industrial |
| 2021-09-08 | The Hospital for Sick Children | Toronto | Ontario | Type II | Medical |
| 2021-09-08 | The Hospital for Sick Children | Toronto | Ontario | Type II | Medical |
| 2021-09-08 | The Hospital for Sick Children | Toronto | Ontario | Type II | Medical |
| 2021-09-10 | Sartell Instrumentation Limited | Mississauga | Ontario | Type II | Commercial |
| 2021-09-10 | Sartell Instrumentation Limited | Mississauga | Ontario | Type II | Commercial |
| 2021-09-10 | Manitoba Infrastructure | Dauphin | Manitoba | Type II | Industrial |
| 2021-09-10 | Manitoba Infrastructure | Russell | Manitoba | Type II | Industrial |
| 2021-09-10 | Manitoba Infrastructure | Snow Lake | Manitoba | Type II | Industrial |
| 2021-09-10 | The Pepsi Bottling Group (Canada), ULC | Mississauga | Ontario | Type II | Industrial |
| 2021-09-14 | Shelby Engineering Ltd. | Sherwood Park | Alberta | Type II | Industrial |
| 2021-09-14 | Uniroc Inc. | Mirabel | Quebec | Type II | Industrial |
| 2021-09-14 | Uniroc Inc. | Mirabel | Quebec | Type II | Industrial |
| 2021-09-14 | Groupe MC2 Inc. | Pointe-aux-Trembles | Quebec | Type II | Industrial |
| 2021-09-15 | Groupe ABS Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-09-15 | Groupe ABS Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-09-15 | SNC-Lavalin GEM Québec Inc. | Montréal | Quebec | Type II | Industrial |
| 2021-09-15 | MR Engineering Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-09-15 | Soil-Mat Engineers & Consultants Ltd. | Hamilton | Ontario | Type II | Industrial |
| 2021-09-15 | Mistras Canada, Inc. | Calgary | Alberta | Type II | Industrial |
| 2021-09-15 | Buffalo Inspection Services (2005) Inc. | Three Hills | Alberta | Type II | Industrial |
| 2021-09-15 | Bunge Canada Holdings I Inc. | Hamilton | Ontario | Type II | Industrial |
| 2021-09-16 | Sintra Inc. | Saint-Isidore | Quebec | Type II | Industrial |
| 2021-09-16 | EXP Services Inc. / Les Services EXP Inc. | Granby | Quebec | Type II | Industrial |
| 2021-09-17 | Excavation Daniel Latour Inc. | Lavaltrie | Quebec | Type II | Industrial |
| 2021-09-17 | Atlantic Packaging Products Ltd. | Scarborough | Ontario | Type II | Industrial |
| 2021-09-18 | TTES Consulting Inc. | MacGregor | Manitoba | Type II | Industrial |
| 2021-09-18 | TTES Consulting Inc. | Reston | Manitoba | Type II | Industrial |
| 2021-09-18 | TTES Consulting Inc. | Brandon | Manitoba | Type II | Industrial |
| 2021-09-20 | Parkland Geotechnical Consulting Ltd. | Red Deer | Alberta | Type II | Industrial |
| 2021-09-20 | Sunnybrook Health Sciences Centre | Toronto | Ontario | Type II | Medical |
| 2021-09-20 | Sunnybrook Health Sciences Centre | Toronto | Ontario | Type II | Medical |
| 2021-09-20 | Sunnybrook Health Sciences Centre | Toronto | Ontario | Type II | Medical |
| 2021-09-21 | Parkland Geotechnical Consulting Ltd. | Red Deer | Alberta | Type II | Industrial |
| 2021-09-21 | CISSS Bas-Saint-Laurent | Rimouski | Quebec | Type II | Medical |
| 2021-09-22 | York University | Toronto | Ontario | Type II | Academic and research |
| 2021-09-22 | York University | Toronto | Ontario | Type II | Academic and research |
| 2021-09-22 | York University | Toronto | Ontario | Type II | Academic and research |
| 2021-09-22 | York University | Toronto | Ontario | Type II | Academic and research |
| 2021-09-22 | St Lawrence Testing & Inspection Co. Ltd. | Cornwall | Ontario | Type II | Industrial |
| 2021-09-22 | Intertape Polymer Inc. | Cornwall | Ontario | Type II | Industrial |
| 2021-09-23 | R.W. Tomlinson Limited | Ottawa | Ontario | Type II | Industrial |
| 2021-09-23 | McIntosh Perry Consulting Engineers Ltd. | Nepean | Ontario | Type II | Industrial |
| 2021-09-23 | Carp Road Animal Hospital | Stittsville | Ontario | Type II | Medical |
| 2021-09-24 | Lascelles Engineering and Associates Ltd. | Hawkesbury | Ontario | Type II | Industrial |
| 2021-09-24 | Sonoco Flexible Packaging Canada Corporation | Terrebonne | Quebec | Type II | Industrial |
| 2021-09-27 | Englobe Corp. | La Baie | Quebec | Type II | Industrial |
| 2021-09-27 | CanRoof Corporation Inc. | Toronto | Ontario | Type II | Industrial |
| 2021-09-27 | Simmons Pet Food On, Inc. | Mississauga | Ontario | Type II | Industrial |
| 2021-09-27 | Maksteel Holdings ULC | Mississauga | Ontario | Type II | Industrial |
| 2021-09-28 | CEGEP de Chicoutimi | Chicoutimi | Quebec | Type II | Industrial |
| 2021-09-28 | Cool Beer Brewing Co. Incrporated | Toronto | Ontario | Type II | Industrial |
| 2021-09-28 | Voltage Wireline Inc. | Brooks | Alberta | Type II | Industrial |
| 2021-09-28 | Centre intégré universitaire de santé et de services sociaux | Chicoutimi | Quebec | Type II | Medical |
| 2021-09-28 | Centre intégré universitaire de santé et de services sociaux | Chicoutimi | Quebec | Type II | Medical |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Academic and research |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Academic and research |
| 2021-09-29 | Pavex Ltée | Saint-Félicien | Quebec | Type II | Industrial |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Medical |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Medical |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Medical |
| 2021-09-29 | Unity Health Toronto | Toronto | Ontario | Type II | Medical |
| 2021-10-01 | CMT Engineering Inc. | St. Clements | Ontario | Type II | Industrial |
| 2021-10-04 | Stuart Hunt & Associates Ltd. | Mississauga | Ontario | Type II | Commercial |
| 2021-10-04 | Englobe Corp. | Anjou | Quebec | Type II | Industrial |
| 2021-10-04 | Englobe Corp. | Anjou | Quebec | Type II | Industrial |
| 2021-10-04 | 9395-8049 QuebecC inc. | Repentigny | Quebec | Type II | Industrial |
| 2021-10-05 | Construction & Pavage Portneuf Inc. | St-Marc-Carrières | Quebec | Type II | Industrial |
| 2021-10-05 | Groupe Géos Inc. | Lévis | Quebec | Type II | Industrial |
| 2021-10-05 | Boss Wireline Services Ltd. | Brooks | Alberta | Type II | Industrial |
| 2021-10-06 | Tecsol GM Inc. | Québec | Quebec | Type II | Industrial |
| 2021-10-06 | Trenergy Inc. | St Catharines | Ontario | Type II | Industrial |
| 2021-10-07 | NDT Group Inc. | Brantford | Ontario | Type II | Industrial |
| 2021-10-07 | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rivière-du-Loup | Quebec | Type II | Medical |
| 2021-10-08 | The Graff Company Ltd. | Mississauga | Ontario | Type II | Industrial |
| 2021-10-12 | Certified Testing Systems (2009) Inc. | Kitchener | Ontario | Type II | Industrial |
| 2021-10-13 | West Fraser Mills Ltd. | Hinton | Alberta | Type II | Industrial |
| 2021-10-13 | Spectrum Wireline Services Ltd. | Red Deer County | Alberta | Type II | Industrial |
| 2021-10-13 | Teck Coal Limited | Elkford | British Columbia | Type II | Industrial |
| 2021-10-14 | 9395-8049 QC inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-10-14 | 9395-8049 QC inc. | Saint-Laurent | Quebec | Type II | Industrial |
| 2021-10-14 | FNX-INNOV Inc. | Longueuil | Quebec | Type II | Industrial |
| 2021-10-14 | Trican Well Service Ltd. | Hinton | Alberta | Type II | Industrial |
| 2021-10-14 | Royal Victoria Health Centre | Barrie | Ontario | Type II | Medical |
| 2021-10-15 | Trans Mountain Pipeline ULC | Jasper | Alberta | Type II | Industrial |
| 2021-10-19 | NOVA Chemicals Corporation | Lacombe | Alberta | Type II | Industrial |
| 2021-10-20 | Chief Medical Supplies Ltd. | Mississauga | Ontario | Type II | Industrial |
| 2021-10-21 | Highland Valley Copper | Logan Lake | British Columbia | Type II | Industrial |
| 2021-10-25 | McMaster University | Hamilton | Ontario | Type II | Academic and Research |
| 2021-10-25 | MWM Consulting Inc. | Saint John | New Brunswick | Type II | Industrial |
| 2021-10-25 | RTD Quality Services Inc. | Saint John | New Brunswick | Type II | Industrial |
| 2021-10-26 | Construction & Pavage Portneuf Inc. | St-Marc-Carrières | Quebec | Type II | Industrial |
| 2021-10-26 | Maskimo Construction Inc. | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-10-26 | FNX-INNOV Inc. | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-10-26 | Custom Fabricators & Machinists Limited / Fabricants et Mach | Dartmouth | Nova Scotia | Type II | Industrial |
| 2021-10-26 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Medical |
| 2021-10-26 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Medical |
| 2021-10-26 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Medical |
| 2021-10-26 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Medical |
| 2021-10-26 | Pet Focus Veterinary Group Inc. | Dartmouth | Nova Scotia | Type II | Medical |
| 2021-10-27 | Centre for Probe Development and Commercialization / | Hamilton | Ontario | Type II | Commercial |
| 2021-10-27 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Commercial |
| 2021-10-27 | Nova Scotia Health Authority | Halifax | Nova Scotia | Type II | Commercial |
| 2021-10-27 | GHD Consultants Ltd. | Chicoutimi | Quebec | Type II | Industrial |
| 2021-10-27 | Harbourside Geotechnical Consultants Limited | Dartmouth | Nova Scotia | Type II | Industrial |
| 2021-10-27 | Canadoil Forge Ltée/Canadoil Forge Ltd. | Bécancour | Quebec | Type II | Industrial |
| 2021-10-27 | Intertape Polymer Inc. | Truro | Nova Scotia | Type II | Industrial |
| 2021-10-27 | Commandite Kruger Wayagamack Inc. | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-10-27 | McMaster University | Hamilton | Ontario | Type II | Medical |
| 2021-10-28 | St-Isidore Asphalte Ltée | St-Isidore | New Brunswick | Type II | Industrial |
| 2021-10-28 | Le Groupe Roy Consultants Ltee | Bathurst | New Brunswick | Type II | Industrial |
| 2021-10-28 | Integrated Sustainability Consultants Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-10-28 | Gemtec Consulting Engineers and Scientists Limited | Bathurst | New Brunswick | Type II | Industrial |
| 2021-10-28 | Les Laboratoires d'Essais Mequaltech Inc. | Bécancour | Quebec | Type II | Industrial |
| 2021-10-28 | Trevali Mining (New Brunswick) Ltd. | Bathurst | New Brunswick | Type II | Industrial |
| 2021-10-28 | Commandité Kruger Trois-Rivières Inc. | Trois-Rivières | Quebec | Type II | Industrial |
| 2021-11-02 | Agriculture Canada | Ste-Hyacinthe | Quebec | Type II | Industrial |
| 2021-11-02 | Mistras Canada, Inc. | Sherwood Park | Alberta | Type II | Industrial |
| 2021-11-05 | SNC-Lavalin GEM Québec Inc. | Laval | Quebec | Type II | Industrial |
| 2021-11-05 | SNC-Lavalin GEM Québec Inc. | Laval | Quebec | Type II | Industrial |
| 2021-11-08 | University Health Network | Toronto | Ontario | Type II | Medical |
| 2021-11-09 | Mezei Inspections Ltd. | Drayton Valley | Alberta | Type II | Industrial |
| 2021-11-09 | Recon Petrotechnologies Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-11-10 | ROFS Canada Ltd. | Blackfalds | Alberta | Type II | Industrial |
| 2021-11-10 | Yemsol Ltd. | Edson | Alberta | Type II | Industrial |
| 2021-11-10 | Galey Inspection Services Ltd. | Parkland County | Alberta | Type II | Industrial |
| 2021-11-10 | Galey Inspection Services Ltd. | Sexsmith | Alberta | Type II | Industrial |
| 2021-11-10 | ROFS Canada Ltd. | Red Deer | Alberta | Type II | Industrial |
| 2021-11-15 | Candu Energy Inc. | Whitby | Ontario | Type II | Commercial |
| 2021-11-16 | Kinectrics Inc | Teeswater | Ontario | Type II | Commercial |
| 2021-11-16 | Inline Group Inc. | Kitimat | British Columbia | Type II | Industrial |
| 2021-11-16 | Inline Group Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-11-18 | Spectris Canada Inc. | St-Laurent | Quebec | Type II | Commercial |
| 2021-11-18 | Golder Associates Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-11-18 | Cytec Canada Inc. | Niagara Falls | Ontario | Type II | Industrial |
| 2021-11-18 | Sleeman Breweries Ltd. | Guelph | Ontario | Type II | Industrial |
| 2021-11-19 | McMaster University | Hamilton | Ontario | Type II | Academic and Research |
| 2021-11-19 | McMaster University | Hamilton | Ontario | Type II | Academic and Research |
| 2021-11-19 | McMaster University | Hamilton | Ontario | Type II | Academic and Research |
| 2021-11-19 | McMaster University | Hamilton | Ontario | Type II | Academic and Research |
| 2021-11-19 | McMaster University | Hamilton | Ontario | Type II | Commercial |
| 2021-11-19 | Shad & Associates Inc. | Vaughan | Ontario | Type II | Industrial |
| 2021-11-19 | Geomaple geotechnics Inc. | North York | Ontario | Type II | Industrial |
| 2021-11-22 | CHU de Québec – Université Laval | Québec | Quebec | Type II | Medical |
| 2021-11-22 | CHU de Québec – Université Laval | Québec | Quebec | Type II | Medical |
| 2021-11-23 | Cox Construction Limited | Guelph | Ontario | Type II | Industrial |
| 2021-11-23 | Magna Exteriors Inc. | Guelph | Ontario | Type II | Industrial |
| 2021-11-23 | Canada Border Services Agency | Ottawa | Ontario | Type II | Industrial |
| 2021-11-24 | University of Ontario Institute of Technology | Oshawa | Ontario | Type II | Academic and research |
| 2021-11-24 | Davroc Testing Laboratories Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-11-24 | Davroc Testing Laboratories Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-11-24 | Davroc Testing Laboratories Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-11-24 | Gunron Inspections Ltd. | Dawson Creek | British Columbia | Type II | Industrial |
| 2021-11-24 | Gunron Inspections Ltd. | Yellowhead County | Alberta | Type II | Industrial |
| 2021-11-24 | Polar Pak Inc. | Brampton | Ontario | Type II | Industrial |
| 2021-11-24 | Centre de santé et de services sociaux de Sept-Îles | Sept-Iles | Quebec | Type II | Medical |
| 2021-11-24 | William Osler Health Centre | Brampton | Ontario | Type II | Medical |
| 2021-11-24 | Centre de santé et de services sociaux de Sept-Îles | Sept-Iles | Quebec | Type II | Medical |
| 2021-11-24 | William Osler Health Centre | Brampton | Ontario | Type II | Medical |
| 2021-11-25 | Lakeridge Health | Oshawa | Ontario | Type II | Medical |
| 2021-11-25 | Lakeridge Health | Oshawa | Ontario | Type II | Medical |
| 2021-11-26 | Petro-Canada Lubricants Inc. | Mississauga | Ontario | Type II | Industrial |
| 2021-11-26 | Petro-Canada Lubricants Inc. / Lubrifiants Petro-Canada Inc. | Mississauga | Ontario | Type II | Industrial |
| 2021-11-26 | CHU de Québec – Université Laval | Québec City | Quebec | Type II | Medical |
| 2021-11-26 | CHU de Québec – Université Laval | Québec City | Quebec | Type II | Medical |
| 2021-11-29 | University of Western Ontario | London | Ontario | Type II | Academic and research |
| 2021-11-29 | Centre intégré de santé et de services sociaux de Trois-Rivières | Trois-Rivières | Quebec | Type I | Medical |
| 2021-11-29 | Provincial Health Services Authority | Kelowna | British Columbia | Type II | Medical |
| 2021-11-30 | Weatherford Canada Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-11-30 | Weatherford Canada Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-11-30 | Weatherford Canada Ltd. | Nisku | Alberta | Type II | Industrial |
| 2021-11-30 | London Health Sciences Centre | London | Ontario | Type II | Medical |
| 2021-11-30 | QEII Health Sciences Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-11-30 | QEII Health Sciences Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-11-30 | QEII Health Sciences Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-11-30 | QEII Health Sciences Centre | Halifax | Nova Scotia | Type II | Medical |
| 2021-12-01 | Task-Master Inspections Ltd. | Fort Saskatchewan | Alberta | Type II | Industrial |
| 2021-12-01 | Provincial Health Services Authority | Prince George | British Columbia | Type II | Medical |
| 2021-12-01 | Toronto Equine Hospital | Mississauga | Ontario | Type II | Medical |
| 2021-12-02 | St. Joseph's Health Care, London | London | Ontario | Type II | Commercial |
| 2021-12-02 | 2709081 Ontario Limited | Tottenham | Ontario | Type II | Industrial |
| 2021-12-09 | Isologic Innovative Radiopharmaceuticals Ltd. | Montréal | Quebec | Type II | Commercial |
| 2021-12-09 | GeoPacific Consultants Ltd. | Kamloops | British Columbia | Type II | Industrial |
| 2021-12-09 | 2141478 Alberta Ltd. | Edmonton | Alberta | Type II | Industrial |
| 2021-12-09 | Central Alberta Medical Imaging Services Limited | Red Deer | Alberta | Type II | Medical |
| 2021-12-14 | 42256 Yukon Inc. | Edmonton | Alberta | Type II | Industrial |
| 2021-12-14 | Valbruna ASW Inc. | Welland | Ontario | Type II | Industrial |
| 2021-12-15 | FNX-INNOV Inc. | Baie-Comeau | Quebec | Type II | Industrial |
| 2021-12-15 | Agrium Inc. | Redwater | Alberta | Type II | Industrial |
| 2021-12-16 | Cepsa Chimie Bécancour Inc. / Cepsa Química Bécancour Inc. | Bécancour | Quebec | Type II | Industrial |
| 2021-12-16 | Beta Research Laboratories Ltd. | Calgary | Alberta | Type II | Industrial |
| 2021-12-17 | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | Ontario | Type II | Commercial |
| 2021-12-20 | BWXT | Kanata | Ontario | Type II | Industrial |
| 2021-12-21 | Mevex Corporation | Stittsville | Ontario | Type II | Commercial |
Appendix G: Stakeholder engagement activities in 2021
| Date | Audience / Meeting attendees | Type of activity | Topics |
|---|---|---|---|
| January 2021 | Medical physicists | Article in InterACTIONS, newsletter of the Canadian Organization of Medical Physicists |
|
| January 2021 | CNSC, Canadian Radiation Protection Association, Canadian Organization of Medical Physicists | C3 Working Group virtual meeting |
|
| February 2021 | Métis Nation of Ontario | Virtual meeting |
|
| March 2021 | Public | Virtual meeting |
|
| March 2021 | CNSC, Canadian Radiation Protection Association | CRPA-CNSC Working Group virtual meeting |
|
| March 2021 | Accelerator and Class II facility licensees | Virtual town hall |
|
| April 2021 | Medical physicists | Article in InterACTIONS, newsletter of the Canadian Organization of Medical Physicists |
|
| May 2021 | Licensees possessing an Elekta linear accelerator | Targeted email |
|
| May 2021 | CNSC, Canadian Radiation Protection Association (CRPA), Canadian Organization of Medical Physicists | C3 Working Group virtual meeting |
|
| May 2021 | Saugeen Ojibway Nation | Virtual meeting |
|
| June 2021 | Federal Authority for Nuclear Regulation – United Arab Emirates | Virtual meeting |
|
| June 2021 | Portable gauge licensees | Targeted email |
|
| June 2021 | Petroleum Services Association of Canada and Energy Safety Canada | Virtual meeting |
|
| June 2021 | Accelerator and Class II facility licensees | Virtual town hall |
|
| June 2021 | Medical physicists | Presentation at the Canadian Organization of Medical Physicists conference |
|
| June 2021 | Medical physicists | Presentation of CNSC staff member’s research at the Canadian Organization of Medical Physicists conference |
|
| July 2021 | Medical physicists | Article in InterACTIONS, newsletter of the Canadian Organization of Medical Physicists |
|
| July 2021 | Portable gauge licensees | Targeted email |
|
| August 2021 | Portable gauge licensees | Targeted email |
|
| September 2021 | All DNSR licensees that have thyroid monitoring conditions included on their licence | Targeted email |
|
| September 2021 | Fixed gauge licensees that do vessel or hopper entry | Targeted email |
|
| September 2021 | Cyclotron radiation safety officers | Virtual town hall |
|
| October 2021 | Medical physicists | Article in InterACTIONS, newsletter of the Canadian Organization of Medical Physicists |
|
| October 2021 | Members of the public | Virtual meetings (1 session in English, 1 session in French) |
|
| November 2021 | Portable gauge licensees | Targeted email |
|
| November 2021 | CNSC, Canadian Radiation Protection Association, Canadian Organization of Medical Physicists | C3 Working Group virtual meeting |
|
| December 2021 | Accelerators and Class II facility licensees | Virtual town hall |
|
| December 2021 | Portable gauge licensees | Targeted email |
|
| February, May and December 2021 | Industrial Radiography Working Group (industry reps and CNSC staff) | Virtual meetings |
|
| Monthly in 2021 | All DNSR licensees | Targeted emails | Topics covered in the DNSR Digest in 2021:
|
*No formal outreach activities were conducted for WNSLs as they are a small subsector – information is best disseminated to licensees on an individual basis.
**Staff also participate in various international meetings and conferences to share the Canadian/CNSC perspective on topics of interest.
Appendix H: Blank inspection worksheet
Type II Inspection Worksheet PDF (108KB)
Type II Inspection Worksheet
Use Type: 811 – portable gauges
Licensee:
Licence Number:
Address:
City:
Province:
Postal Code:
Phone Number:
Report Number:
Inspection Date:
Inspector Name:
Use Type Number:
Risk Group:
Person Seen:
Abbreviations:
LC : Licence Condition
NSCA : Nuclear Safety and Control Act
GN : General Nuclear Safety and Control Regulations
RP : Radiation Protection Regulations
NSRD : Nuclear Substances and Radiation Devices Regulations
CII : Class II Nuclear Facilities and Prescribed Equipment Regulations
TDG : Transportation of Dangerous Goods Regulations
PTNS : Packaging and Transport of Nuclear Substances Regulations, 2015
SSR-6 : International Atomic Energy Agency (IAEA) Regulations for the Safe Transport of Radioactive Material
SCA: Safety and Control Area
| Seq | SCA | Regulatory Requirement | Description | Compliance Expectations | Risk | Rating | Comments |
|---|---|---|---|---|---|---|---|
| 1. | Radiation protection | LC 2575-2 | Storage | (a)Access to storage areas containing nuclear substances or radiation devices is restricted to authorized personnel. (b)Dose rates at occupied areas outside storage areas do not exceed 2.5 µSv/hr. (c)Dose limits are not exceeded as a result of nuclear substances or radiation devices in storage. | H | Completed by inspector | Completed by inspector |
| 2. | Radiation Protection | NSRD 20 | Meter calibrated | Survey meter that is used has been calibrated within the previous twelve months of its use. | H | Completed by inspector | Completed by inspector |
| 3. | Radiation protection | RP 04(a) | ALARA/RP program | The licensee has implemented a radiation protection program that keeps doses ALARA and includes: (i) management control over work practices; (ii) personnel qualification and training; (iii) control of occupational and public exposure to radiation; and (iv) planning for unusual situations. | H | Completed by inspector | Completed by inspector |
| 4. | Radiation protection | RP 05 | Ascertainment and recording of doses | (1) Personnel doses are ascertained and recorded. (2) Doses are determined by (a) direct measurement or (b) estimation. | H | Completed by inspector | Completed by inspector |
| 5. | Radiation protection | RP 13(1) | Dose limits/body | Dose limits not exceeded. | H | Completed by inspector | Completed by inspector |
| 6. | Radiation protection | RP 20 | Container/Device labelled | Each container or device containing greater than one Exemption Quantity of nuclear substance(s) is labelled with the radiation warning symbol and the required wording. | H | Completed by inspector | Completed by inspector |
| 7. | Radiation protection | RP 21 | Posting of signs | A radiation warning symbol is posted: (a)at the boundary of and at every point of access where there is more than 100 times the Exemption Quantity (EQ) of nuclear substances; or (b)where the radiation dose rate could exceed 0.025 mSv/h. | H | Completed by inspector | Completed by inspector |
| 8. | Radiation protection | LC 2922 | Survey meter availability | Provisions have been made to ensure a survey meter can be available to workers at any site where a radiation device is used, within 2 hours. | M | Completed by inspector | Completed by inspector |
| 9. | Radiation protection | RP 22 | Radiation warning sign | When a radiation warning symbol is used, it is posted in accordance with regulations. | L | Completed by inspector | Completed by inspector |
| 10. | Emergencies and unplanned events | GN 29 | Reportable events | Incidents and unplanned events have been immediately reported to the CNSC and a detailed written report was submitted within 21 days (refer to NSRD 38). | H | Completed by inspector | Completed by inspector |
| 11. | Emergencies and unplanned events | NSRD 21 | Device accidents | Any radiation device involved in an accident or incident has been tested/inspected and confirmed to be functioning properly prior to return to use. | H | Completed by inspector | Completed by inspector |
| 12. | Emergencies and unplanned events | NSRD 22 | Field devices ID | Device is labelled with contact information including a 24 hour telephone number. | H | Completed by inspector | Completed by inspector |
| 13. | Emergencies and unplanned events | NSRD 23 | Contact details posted | The name or job title and a 24 hr. telephone number are posted in a readily visible location where the nuclear substance is stored or used (refer to RP 21). | H | Completed by inspector | Completed by inspector |
| 14. | Emergencies and unplanned events | NSRD 17 | Radiation safety | Referenced emergency procedures are available to workers at the site of licensed activity. | M | Completed by inspector | Completed by inspector |
| 15. | Emergencies and unplanned events | NSRD 18 (3) | Failed leak test | Appropriate actions were taken upon detection of a leaking source. | M | Completed by inspector | Completed by inspector |
| 16. | Emergencies and unplanned events | NSRD 18(1) (c) | Leak test/event | Leak testing was performed immediately after any event that may have damaged the sealed source(s). | L | Completed by inspector | Completed by inspector |
| 17. | Training and Qualification | GN 12(1)(a)(b) | Training and sufficient workers | There are (a) a sufficient number of trained and (b) qualified workers to carry on licensed activity | M | Completed by inspector | Completed by inspector |
| 18. | Training and Qualification | RP 07 | Nuclear energy workers informed | (1)Each NEW has been informed in writing of their NEW designation, of the risks associated with their work, of the M regulatory dose limits and of their individual dose. (2)Female NEW has been informed in writing of their rights (RP 07) and obligations (RP 11). (3)A signed acknowledgment form is available for each NEW. | M | Completed by inspector | Completed by inspector |
| 19. | Operational procedures | GN 12 (1) (e) | Use of equipment and procedures | Licensee ensures equipment, clothing and procedures are used appropriately at the site of the licensed activity. | H | Completed by inspector | Completed by inspector |
| 20. | Operational procedures | GN 13 | Authorized transfer | All transfers of nuclear substances or radiation devices have been done to authorized licensees. | H | Completed by inspector | Completed by inspector |
| 21. | Operational procedures | GN 17 | Worker’s obligations | Every worker: (a)uses equipment, devices, facilities and clothing in a responsible and reasonable manner in accordance with the Act, Regulations and Licence Conditions; (b)complies with procedures and measures established by the licensee; (c) informs the licensee or supervisor of any situation where there may be: (i)an increase in the risk to the environment or the health and safety of persons; (ii)a threat to security; (iii)a failure to comply with regulatory requirements; (iv)sabotage, theft, loss or illegal use or possession of prescribed equipment, or (v)a release into the environment not authorized by the licence; (d)observes and obeys all notices and warning signs; and (e)takes all reasonable precautions to ensure the safety and security of individuals, the environment and the nuclear substances or facilities. | H | Completed by inspector | Completed by inspector |
| 22. | Operational procedures | LC 2480 | Import export restrictions | The licensee is not authorized to import or export all items described in the schedule, Parts A and B, of the Nuclear Non-proliferation Import and Export Control Regulations, and specifically listed in the licence condition. | H | Completed by inspector | Completed by inspector |
| 23. | Operational procedures | NSRD 11 | Device certification and transfer | (1)The radiation device in use is a certified model (unless authorized in the licence). (2)The radiation device transferred to other licensees is a certified model. | H | Completed by inspector | Completed by inspector |
| 24. | Operational procedures | RP 08 | Licensed dosimetry | A licensed dosimetry service is used where the effective dose of a NEW will likely exceed 5 mSv in a one-year period. | H | Completed by inspector | Completed by inspector |
| 25. | Operational procedures | GN 12(1)(d) | Device provided and maintained | Required devices have been provided and have been maintained according to manufacturer’s instruction. | M | Completed by inspector | Completed by inspector |
| 26. | Operational procedures | LC 2093 | Maintenance limitations | Maintenance is limited to cleaning and lubrication in accordance with the manufacturer's instructions. | M | Completed by inspector | Completed by inspector |
| 27. | Operational procedures | NSRD 36(1)(a) | Inventory | NSRD 36 (1) (a) A complete nuclear substance and radiation device inventory is available. | M | Completed by inspector | Completed by inspector |
| 28. | Operational procedures | NSRD 36(1)(b)(d) (2) | Worker records retained | (1)(b) The name of each worker who handles nuclear substances and/or radiation devices is recorded. (1)(d) Training records for all workers who handle nuclear substances and/or radiation devices are available. (2) Worker training records are kept on file for three years after termination. | M | Completed by inspector | Completed by inspector |
| 29. | Operational procedures | GN 14 | Post licence | (1) A copy of the licence or an appropriate notice is posted in a conspicuous place at the site of the licensed activity. (2) The complete licence is available at field locations | L | Completed by inspector | Completed by inspector |
| 30. | Operational procedures | GN 28 | Records retained | The CNSC was notified 90 days prior to the disposal of any prescribed records. | L | Completed by inspector | Completed by inspector |
| 31. | Operational procedures | LC 2917 | Operation limitations - general | Activities and procedures, as listed in the licence appendix, are followed. | L | Completed by inspector | Completed by inspector |
| 32. | Operational procedures | LC 2920 | Inaccuracies notification | Changes to documents listed in the licence appendix have been reported to the CNSC. | L | Completed by inspector | Completed by inspector |
| 33. | Operational procedures | NSRD 18(1)(a)(b)(d) | Leak test | Leak testing is performed at the required frequency following acceptable procedures. | L | Completed by inspector | Completed by inspector |
| 34. | Operational procedures | NSRD 19 | Transfer documents | (1)A copy of the most recent leak test result is provided for all transfers of radiation devices as well as instructions to follow in the event of an accident. (2)A copy of the most recent leak test result is provided for all transfers of sealed source or nuclear substance used as shielding. | L | Completed by inspector | Completed by inspector |
| 35. | Operational procedures | NSRD 36(1) (c)(e),(3),(4) | Records retained | (1)(c) Records of transfer, receipt, disposal and abandonment are available. (1)(e) Records of inspection, measurement, test and servicing are available. (3), (4) Records of inspection, measurement, test and servicing are kept on file for three years. | L | Completed by inspector | Completed by inspector |
| 36. | Operational procedures | RP 23 | Frivolous posting of signs | Radiation warning symbols are not posted where there is no radiation, nuclear substance or prescribed equipment. | L | Completed by inspector | Completed by inspector |
| 37. | Operational procedures | RP 24 | List of NEWs | A record including names and job category of each NEW is available. | L | Completed by inspector | Completed by inspector |
| 38. | Organization and management | NSCA 26 | Licence details | Licence activities are conducted in accordance with the licence. | H | Completed by inspector | Completed by inspector |
| 39. | Organization and management | GN 15(c) | Change notified | Changes of personnel responsible for management and control of licensed activity (RSO, Applicant Authority and Signing Authority) have been reported to the CNSC within 15 days. | M | Completed by inspector | Completed by inspector |
| 40. | Organization and management | LC 2300 | Location notification | CNSC was informed in writing, within seven days, of sites where licensed activities were conducted for more than 90 days. Discontinuance of such sites was also reported within 7 days. | M | Completed by inspector | Completed by inspector |
| 41. | Organization and management | LC 2916 | Annual compliance report | The licensee submits the annual compliance report in the form specified in the appendix of the licence for each year the licence is valid. | M | Completed by inspector | Completed by inspector |
| 42. | Organization and management | GN 12(1)(k) | Act/Regs available | A copy of the Act and Regulations (paper or electronic copy) are readily available to all workers. | L | Completed by inspector | Completed by inspector |
| 43. | Organization and management | LC 2350 | Record requirements (>90 day at sites) | Records and operational procedures are available at storage/use locations (greater than 90 consecutive days). | L | Completed by inspector | Completed by inspector |
| 44. | Security | GN 12(1)(c)(g)(h)(i)(j) | Security indicators | Provisions are in place to ensure the security of nuclear substances and radiation devices and the health and safety of persons. This may be achieved through restricted access (for example use of locks, alarms, and security systems) and reporting of incidents including loss, theft and sabotage. | H | Completed by inspector | Completed by inspector |
| 45. | Security | LC 2490 | Sealed source security requirements | Licensees have in place security measures including:
| H | Completed by inspector | Completed by inspector |
| 46. | International obligations/ safeguards | Import restrictions | LC 2402 | Imports are within the limits specified in the licence condition. | H | Completed by inspector | Completed by inspector |
| 47. | International obligations/ safeguards | Export restrictions | LC 2403 | Exports are within the limits specified in the licence condition. | H | Completed by inspector | Completed by inspector |
| 48. | Packaging and transport | Package secured in vehicle | PTNS 25(4) | Consignments are segregated and securely stowed (refer to SSR-6 562, 564, 574 - PTNS 25(1) and TDG 5.4). Category II-Yellow and III-Yellow packages are not carried in compartments occupied by passengers - SSR-6 563. | H | Completed by inspector | Completed by inspector |
| 49. | Packaging and transport | Excepted packages content/activity | PTNS 26(1)(a) | Excepted packages meet the following criteria:
| H | Completed by inspector | Completed by inspector |
| 50. | Packaging and transport | Type A package requirements | PTNS 28(1) | A Type A package must be prepared and labelled in accordance of PTNS 28(1) and associated requirements from SSR-6. Package requirements are as follows:
| Completed by inspector | Completed by inspector | |
| 51. | Packaging and transport | Reporting requirements | PTNS 37,38, 40 | The consignor, the carrier and the consignee must provide an immediate report to CNSC (PTNS 37 (1)) and a 21 day report (PTNS 38) when becoming aware of any of the following situations:
| H | Completed by inspector | Completed by inspector |
| 52. | Packaging and transport | Type A package certification | PTNS 42 | Type A package design, test results and packaging instructions kept on file for two years after last shipment. | H | Completed by inspector | Completed by inspector |
| 53. | Packaging and transport | Showing proof of TDG training | PTNS 25(1) | A person handling dangerous goods must provide their training certificate or copy of it to an inspector immediately upon request. TDG 6.8 This requirement does not apply for excepted package (TDG 1.43 (b)). | M | Completed by inspector | Completed by inspector |
| 54. | Packaging and transport | Competent authority certificates | PTNS 25(2)(c) | Consignor has competent authority certificates for applicable sources and packages (refer to SSR-6 561). | M | Completed by inspector | Completed by inspector |
| 55. | Packaging and transport | Transport document requirement | PTNS 29(1) | The consignor of radioactive material provides a shipping document that includes the following (refer to TDG 3.5 and SSR-6 546):
For consignments of more than one package, the required information (*) must be given for each package.
| M | Completed by inspector | Completed by inspector |
| 56. | Packaging and transport | Shipping doc kept 2 years | TDG 3.11 | Shipping documents used are kept on file for two years. | M | Completed by inspector | Completed by inspector |
| 57. | Packaging and transport | Transport document location | TDG 3.7 | Shipping document is located within driver’s reach or in a door pocket on the driver’s side. | M | Completed by inspector | Completed by inspector |
| 58 | Packaging and transport | TDG training certificate | TDG 6.1, 6.3, 6.5 | The employer is responsible for: 6.1(2)(a) ensuring that only an adequately trained worker who holds a valid TDG certificate handle Class 7 dangerous goods ; or 6.1 (2)(b) performs those activities in the presence and under the direct supervision of a person who is adequately trained and who holds a training certificate in accordance with this Part. 6.3 issuing training certificate that includes:
| M | Completed by inspector | Completed by inspector |
| 59. | Packaging and transport | TDG training certificate on file | TDG 6.6, 6.7 | A copy of the TDG training certificate is kept on file for two years and is available to the inspector. | M | Completed by inspector | Completed by inspector |
Note: CNSC licensees may use this worksheet voluntarily to ascertain the CNSC`s general expectations regarding regulatory requirements. Such requirements would generally be assessed during a Type I and Type II Inspection of licences issued pursuant to the Nuclear Substances and Radiation Devices Regulations. The expectations listed for each regulatory requirement are only provided as a guide. Similar worksheets will be used by CNSC staff for on-site inspections. Inspections, will, however, be carried out on a case-by-case basis in the context of the licensed activities and the circumstances of individual situations. This worksheet is not intended to limit the scope of CNSC inspections or the powers of CNSC inspectors. Licensees should contact the CNSC to obtain information regarding their specific regulatory requirements.
Appendix I: Safety performance rating levels
The following rating levels, as shown in table 22, reflect the transition in rating terminology used by the CNSC. While some inspection reports still use the previous rating levels due to the licensing and compliance system in use, licensees using nuclear substances and radiation devices can expect this transition to take place over time. For the purposes of reporting in this ROR, the previous rating levels are converted to the new rating levels. The rating definitions below were updated in 2021 and endorsed by the CNSC management team. The fully satisfactory rating is no longer used.
| Previous rating level | Description | New rating level | Description |
|---|---|---|---|
| A and B | Meets expectations | SA | Satisfactory |
| C | Improvement is required | BE | Below expectations |
| D | This area is seriously compromised | ||
| E | Breakdown | UA | Unacceptable |
Satisfactory (SA)
Licensee meets all of the following criteria:
- Performance meets CNSC staff expectations
- Licensee non-compliances or performance issues, if any, are not risk-significant
- Any non-compliances or performance issues have been, or are being, adequately corrected
Below Expectations (BE)
One or more of the following criteria apply:
- Performance does not meet CNSC staff expectations
- Licensee has risk-significant non-compliance(s) or performance issue(s)
- Non-compliances or performance issues are not being adequately corrected
Unacceptable (UA)
One or both of the following criteria apply:
- Risk associated with a non-compliance or performance issue is unreasonable
- At least one significant non-compliance or performance issue exists with no associated corrective action
Appendix J: Relevant documents
J.1 Act and regulations
- Nuclear Safety and Control Act
- Administrative Monetary Penalties Regulations
- Class II Nuclear Facilities and Prescribed Equipment Regulations
- General Nuclear Safety and Control Regulations
- Nuclear Substances and Radiation Devices Regulations
- Packaging and Transport of Nuclear Substances Regulations, 2015
- Nuclear Security Regulations
- Radiation Protection Regulations
- Nuclear Non-proliferation Import and Export Control Regulations
- Canadian Nuclear Safety Commission Cost Recovery Fees Regulations
- Transport of Dangerous Goods Act, 1992 (Transport Canada)
- Transportation of Dangerous Goods Regulations (Transport Canada)
J.2 Regulatory documents
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment
- REGDOC-1.5.1, Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment
- REGDOC-1.6.1, Licence Application Guide: Nuclear Substances and Radiation Devices
- REGDOC-1.6.2, Radiation Protection Programs for Nuclear Substances and Radiation Devices Licences
- REGDOC-2.2.2, Personnel Training
- REGDOC-2.2.3, Personnel Certification: Radiation Safety Officers
- REGDOC-2.2.3, Personnel Certification: Exposure Device Operators (and the associated CSA PCP-09, Certified Exposure Device Operator Personnel Certification Guide)
- REGDOC-2.5.5, Design of Industrial Radiography Installations
- REGDOC-2.5.7, Design, Testing and Performance of Exposure Devices
- REGDOC-2.7.1, Radiation Protection
- REGDOC-2.7.2, Dosimetry, Volume I: Ascertaining Occupational Dose
- REGDOC-2.9.1, Environmental Protection: Environmental Principles, Assessments and Protection Measures
- REGDOC-2.11, Framework for Radioactive Waste Management and Decommissioning in Canada
- REGDOC-2.11.1, Waste Management, Volume I: Management of Radioactive Waste
- REGDOC-2.11.2, Decommissioning
- REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources and Category I, II and III Nuclear Material
- REGDOC-2.13.1, Safeguards and Nuclear Material Accountancy
- REGDOC-2.13.2, Import and Export, Version 2
- REGDOC-2.14.1, Volume I: Information Incorporated by Reference in Canada’s Packaging and Transport of Nuclear Substances Regulations, 2015
- REGDOC-3.1.3, Reporting Requirements for Waste Nuclear Substance Licensees, Class II Nuclear Facilities and Users of Prescribed Equipment, Nuclear Substances and Radiation Devices
- REGDOC-3.2.1, Public Information and Disclosure
- REGDOC-3.2.2, Indigenous Engagement
- REGDOC-3.3.1, Financial Guarantees for Decommissioning of Nuclear Facilities and Termination of Licensed Activities
- REGDOC-3.5.2, Compliance and Enforcement: Administrative Monetary Penalties
- REGDOC-3.5.2, Compliance and Enforcement, Volume II: Orders under the Nuclear Safety and Control Act
- REGDOC-3.5.3, Regulatory Fundamentals
- REGDOC-3.6, Glossary of CNSC Terminology
Other relevant documents
Page details
- Date modified: